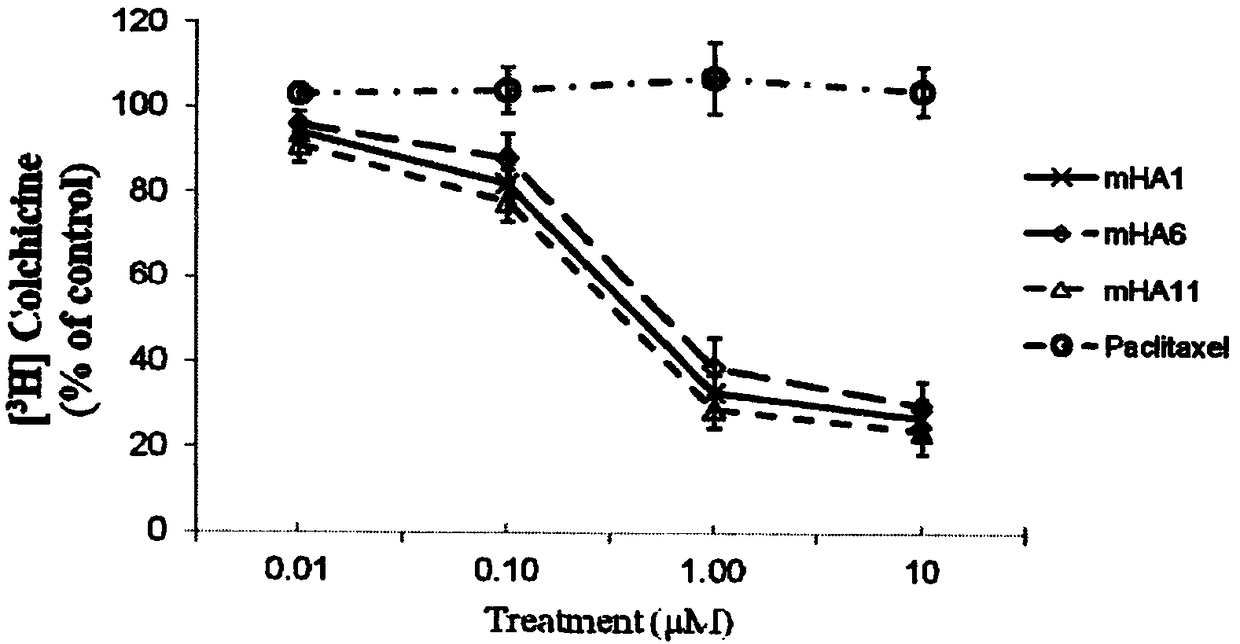
A camphorane compound that inhibits tubulin and its preparation method and application
A tubulin and compound technology, applied in the field of chemistry, can solve problems such as difficult synthesis and complex structure, and achieve the effects of simple preparation method, easy operation, and strong anti-microtubule polymerization effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
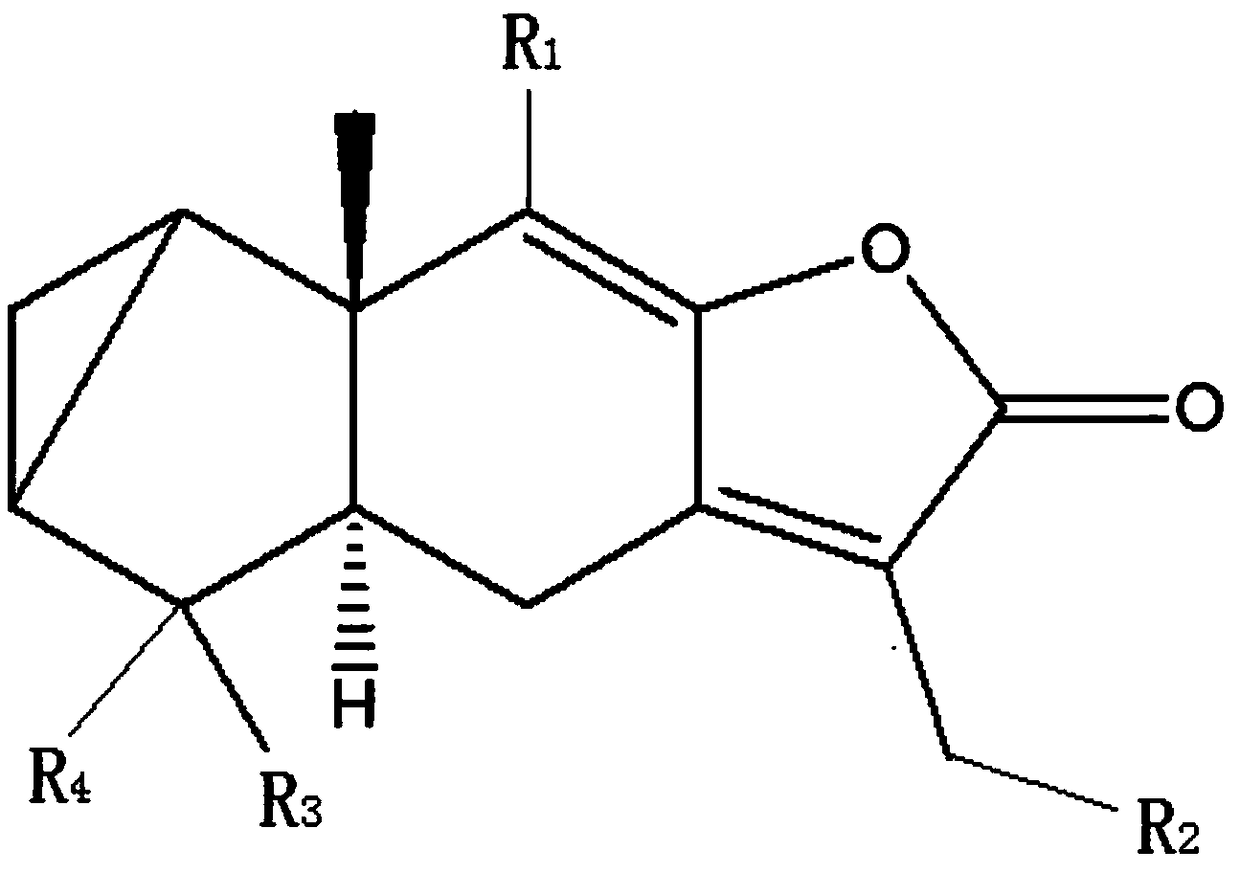
[0031] In the embodiment of the present invention, a camphorane compound that inhibits tubulin, the structural formula of the camphorane compound is as shown in (1):
[0032]
[0033] (1), R in (1) 1 , R 3 Is an alkane group, an alkenyl group, an alkyne group, R 2 , R 4 is H, hydroxyl, alkoxy, amino or halo.
[0034] The preparation method of described camphorane compound, comprises the following steps:
[0035] 1) Preparation of crude extract from chloroform fraction
[0036] The underground part of Chrysanthemum multispike is air-dried in a cool place, then crushed to obtain coarse powder of the medicinal material, using ethanol with a volume fraction of 95% as the extraction solvent, extracting by heating and pressurizing the extraction tank at 70°C until the color of the extract is light, and then extracting together Liquid, use a rotary evaporator to recover the extraction solvent under reduced pressure, and dry it in a 60°C oven to obtain an extract. Dissolve and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com