Preparation method of flufenacet
A technology of flufenacet and flufenacet, which is applied in the field of preparation of flufenacet, can solve the problems of not obtaining yield, increasing the possibility of competitive side reactions, and reducing yield, and achieves that the process conditions are not harsh , the synthesis method is simple, the effect of less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 12-amino-5-trifluoromethyl-1,3,4-thiadiazole
[0035] Thiosemicarbazide (145g, 1.59mol) was suspended in 1L of 1,4-dioxane, stirred mechanically; adding CF 3 COOH (120 mL, 1.59 mol); POCl was added dropwise 3 (150mL, 1.59mol), the temperature rises obviously, after adding in about 30 minutes, the reaction solution first becomes extremely thick and then slightly thinner; the external temperature is 110°C for 3 hours under reflux reaction, a large amount of HCl gas is generated, the reaction solution gradually becomes thinner, and a jelly-like solid settling;
[0036] The reaction solution was allowed to stand for 30 minutes, the supernatant was poured out, 1 L of ice water was added to the residue, and mechanically stirred; the pH was adjusted to 9 with 50% NaOH solution (~2eq, 3.2mol), and a solid was precipitated, and the mother liquor was cooled to 20°C. The solid was obtained by suction filtration, washed once with ice water, and dried...
Embodiment 22
[0037] The preparation of embodiment 22-chloro-5-trifluoromethyl-1,3,4-thiadiazole
[0038] 2-amino-5-trifluoromethyl-1,3,4-thiadiazole (130g, 0.768mol) was suspended in 1.5L of 37wt% hydrochloric acid, mechanically stirred; Cu powder (18.2g, 0.284mol) was added; cold To internal temperature -10°C; add dropwise 350mL containing NaNO 2 (212g, 3.07mol, 4eq) aqueous solution, the temperature is controlled at -10°C to -5°C, and it takes about 2h to complete the addition.
[0039]During the reaction process, brown gas is always generated, and the reaction solution becomes very thick, and a large amount of solids are generated (it is easy to spray if the drop is too fast); then stir at -5°C to 5°C for 1 to 2 hours, and the reaction solution becomes thinner; the temperature rises to 40 ℃~50℃ and then stirred for 2~3 hours, the reaction solution gradually became thinner, and turned into a brown-yellow suspension;
[0040] After cooling to room temperature, CH was added to the reacti...
Embodiment 3
[0043] The preparation of embodiment 3 flufenacet
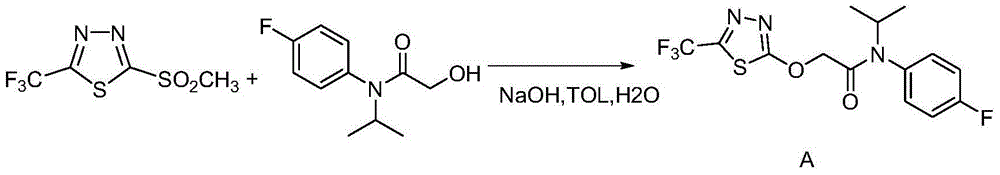
[0044] Potassium tert-butoxide (tBuOK) (2.7g, 0.0236mol) was suspended in 50mL tert-butanol (tBuOH), in a water bath at room temperature (around 25°C); add 2-hydroxy-N-(4-fluoroaniline)-N-( 1-methylethyl) acetamide (5g, 0.0236mol); Add 2-chloro-5-trifluoromethyl-1,3,4-thiadiazole (4.5g, 0.0236mol) dropwise under stirring, react The system will be slightly exothermic and form a light yellow suspension; then stir at room temperature for 1-2 hours until the reaction is complete. The reaction formula is as follows:
[0045]
[0046] Add CH to the reaction solution 2 Cl 2 300mL, washed twice with brine with a concentration of 20%~saturated (with emulsification), took the organic phase to dry, and recovered CH 2 Cl 2 The crude product of flufenacet (8.2 g, 96%) was obtained. TLC basically has no impurities, and the purity is 89% as detected by HPLC.
[0047] The crude product of flufenacet can be refined by continuous rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com