Synthesis method of silver nanoparticles
A silver nanoparticle and synthesis method technology, applied in the field of metal nanoparticle production, can solve the problems of reducing environment and biological hazards, and achieve excellent antibacterial effect, long-term storage stability, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
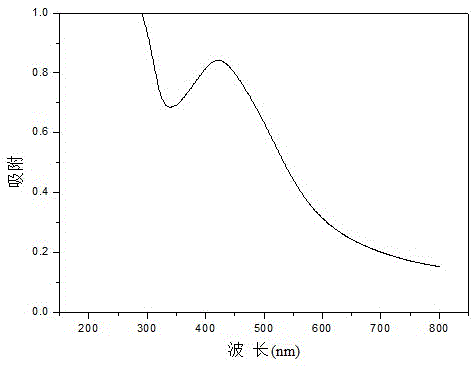
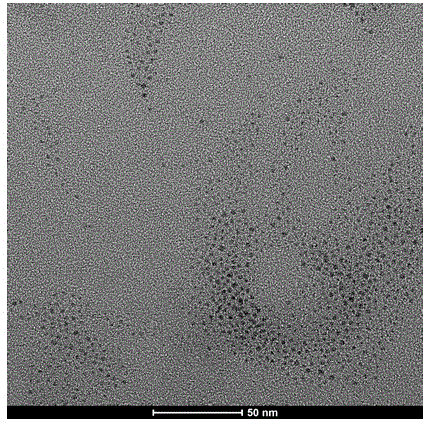
[0024] Add 1mL of 0.1M silver nitrate aqueous solution to 58.0mL of 0.1(wt)% carboxymethyl chitosan aqueous solution. When the above components are uniformly dispersed, add 1mL of 0.1M β-D glucose aqueous solution and continue stirring. The above mixture was reacted at room temperature for 24 h. After reacting for 1 h, the solution turned bright yellow, indicating that silver nanoparticles began to form. The UV-vis adsorption spectrum test result of the final product shows the surface plasmon adsorption of silver elemental particles.
Embodiment 2
[0026] 2 mL of 0.1 M silver nitrate aqueous solution was added to 56.0 mL of 0.1 (wt) % carboxymethyl chitosan aqueous solution. When the above components are uniformly dispersed, add 2mL of 0.1M β-D glucose aqueous solution and continue stirring. The above mixture was reacted at room temperature for 40 h. After reacting for 1 h, the solution turned bright yellow, indicating that silver nanoparticles began to form. The UV-vis adsorption spectrum test result of the final product shows the surface plasmon adsorption of silver elemental particles.
Embodiment 3
[0028] 3 mL of 0.1 M silver nitrate aqueous solution was added to 54.0 mL of 0.15 (wt) % carboxymethyl chitosan aqueous solution. When the above components are evenly dispersed, add 3mL of 0.1ML-glucose aqueous solution and continue stirring. The above mixture was reacted at room temperature for 30 h. After reacting for 1 h, the solution turned bright yellow, indicating that silver nanoparticles began to form. The UV-vis adsorption spectrum test result of the final product shows the surface plasmon adsorption of silver elemental particles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com