A kind of synthetic method of rosuvastatin calcium key intermediate
A technology for the synthesis of rosuvastatin calcium and its synthesis method, which is applied in the field of synthesis of key intermediates of rosuvastatin calcium, can solve the problems that do not conform to the development concept of green chemistry, are not conducive to the health of operators, and are not conducive to large-scale production. Achieve the effects of easy large-scale production, environmental protection, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
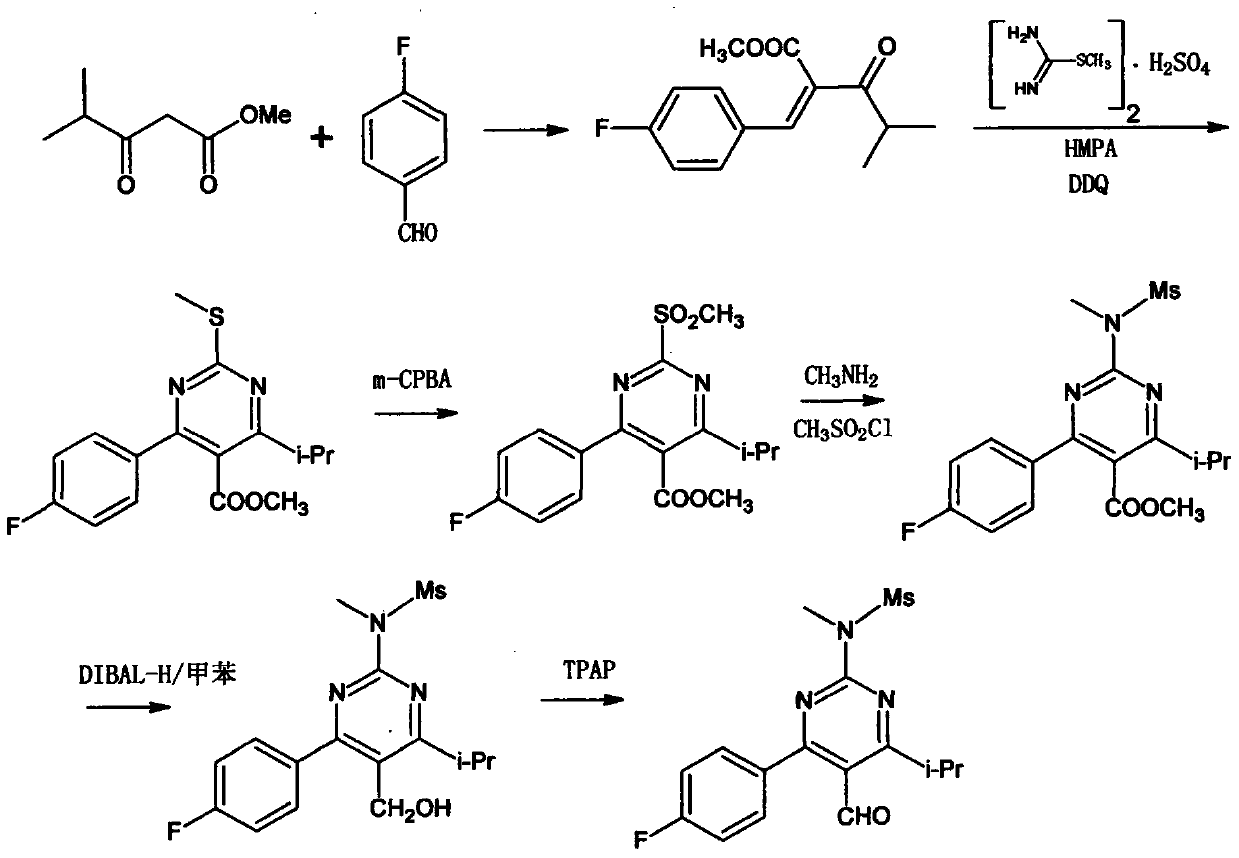
[0034] Example 1: Synthesis of 1-(4-fluorophenyl)-4-methylpentan-1,3-dione (Compound IV)
[0035]
[0036] Add 150g of isopropanol to a 0.5L three-necked flask, then add 6.9g of sodium in batches, stir vigorously and after the sodium is completely dissolved, add 13.81g (0.1mol) of p-fluoroacetophenone and 11.62g of ethyl isobutyrate dropwise (0.1 mol) in 80 g of isopropanol. The reaction solution was refluxed at 82°C for 6 hours, then cooled to room temperature and stirred overnight. A large amount of product was precipitated, filtered to obtain off-white finished product, dried in a blast oven at 40° C. to constant weight, 18 g was obtained, and the yield was 86%. Nuclear magnetic data (1HNMR, 500MHz, internal standard TMS, solvent CDCl3) are as follows: 1.30 (d, J=7.0Hz, 6H, CH 3 ), 2.61(m, 1H, CH), 4.15(s, 1H, CH) 2 ), 7.18-7.12 (m, 2H, Ar-H), 7.93-7.87 (m, 2H, Ar-H).
Embodiment 2
[0037] Example 2: Synthesis of 4-(4-fluorophenyl)-6-isopropyl-N-methylpyrimidin-2-amine (Compound III)
[0038]
[0039] 10.4g (0.05mol) of compound IV, 6g (0.055mol) of methylguanidine hydrochloride, 8.4g (0.15mol) of potassium hydroxide and 100ml of isopropanol were added to a 250ml three-necked flask, and the reaction was carried out under reflux overnight. After the reaction was completed, the isopropanol was distilled off under reduced pressure, naturally cooled to 10° C., filtered, and the filter cake was rinsed with a small amount of isopropanol, and the obtained filter cake was dried in a vacuum oven at 50° C. to constant weight. 11.4 g of off-white solids were obtained with a yield of 93%. Nuclear magnetic data (1HNMR, 500MHz, internal standard TMS, solvent DMSO) are as follows: 1.25 (d, J=6.8Hz, 6H, CH 3 ), 2.98(d, 3H, CH 3 ), 3.18(m, 1H, CH), 4.98(s, 1H, NH), 6.71(s, 1H, Ar-H), 7.16~7.10(m, 2H, Ar-H), 7.52~7.47(m, 2H, Ar-H).
Embodiment 3
[0040] Example 3: Synthesis of 4-(4-fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methanesulfonyl)amino]pyrimidine (Compound II)
[0041]
[0042]Under nitrogen protection, 9.8 g (0.04 mol) of compound III and 100 ml of dichloromethane were added to a 250 ml three-necked flask, and cooled to 5°C. , 12.1 g (0.12 mol) of triethylamine was added, and the reaction was stirred for half an hour. A solution of 5.5 g (0.048 mol) of methanesulfonyl chloride dissolved in 5 ml of dichloromethane was slowly added dropwise to the reaction solution, and the reaction was continued to be stirred at 0 to 25° C. for 12 h. After the reaction was completed, 20 ml of dichloromethane and 30 ml of purification were added, the pH was adjusted to 2-3 with concentrated hydrochloric acid, and the organic layer was obtained by layering. The obtained organic layer was washed once with 50 ml of saturated brine, dried with 10 g of anhydrous sodium sulfate, and filtered. The dichloromethane was removed by pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com