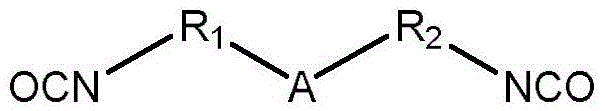
Stimulation sensitive multifunctional polyisocyanate with active groups
A polyisocyanate and active group technology, applied in the field of polyisocyanate, can solve the problem of no multi-functional polyisocyanate, etc., and achieve the effect of wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
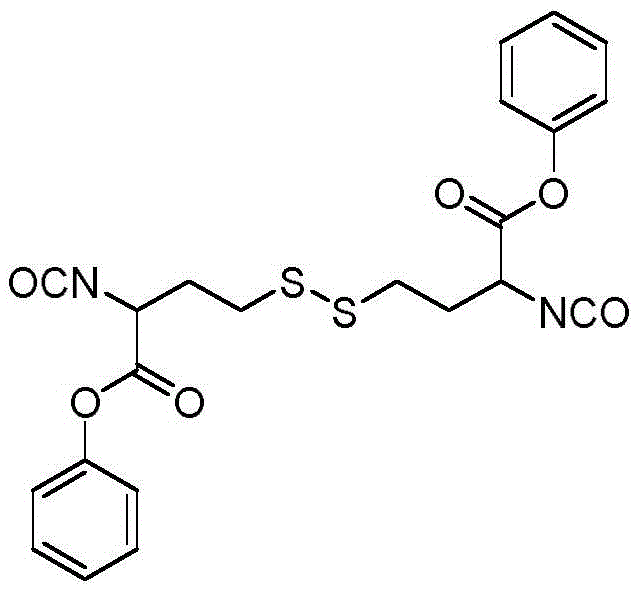
[0025] In this example, the stimulus-sensitive multifunctional polyisocyanate I with active groups was synthesized.
[0026] Dissolve 14.5 g of cystine dimethyl ester in 400 ml of ethyl acetate, and add 10.7 g of triphosgene under nitrogen protection, react at room temperature for 1 hour, and then heat to reflux for 4 hours. After the reaction solution was cooled, it was washed twice with dilute hydrochloric acid, and once with saturated sodium chloride solution and distilled water. The organic phase was dried over anhydrous magnesium sulfate for 4 hours, filtered with suction and concentrated under reduced pressure. The structural formula of the product obtained is:
[0027]
[0028] The infrared spectrum data are as follows:
[0029] FTIR (KBr, cm -1 ): 2957 (s, νCH), 1441 (m, δCH), 2272 (s, vN=C=O), 1746 (s, vC=O).
[0030] The H NMR spectrum data are as follows:
[0031] 1 HNMR (400MHz, CDCl 3 ): δ4.40(q, J=4.2, NCO-CH-, 2H), 3.86(s, CH 3 O-,6H),3.20(dd,J=4.2,9....
Embodiment 2
[0035] In this example, the stimulus-sensitive multifunctional polyisocyanate II with active groups was synthesized.
[0036] Dissolve 8.41 g of dibenzyl homocystine in a mixed solvent of 200 ml of dichloromethane and 200 ml of saturated aqueous sodium bicarbonate solution, cool to 0°C and vigorously stir for 10 minutes. Then the stirring was stopped, and 41 milliliters of phosgene in toluene (1.93 moles per liter) was injected into the organic phase, and stirred for 10 minutes under ice-bath cooling. The reaction solution was layered, the organic phase was washed twice with 0.5 mol per liter of brine, once with distilled water, and the aqueous phase was backwashed once with dichloromethane. After the organic phases were combined, they were dried with anhydrous sodium sulfate for 10 hours, and decompressed after suction filtration. concentrate. The structural formula of the product obtained is:
[0037]
[0038] The infrared spectrum data are as follows:
[0039] FTIR (K...
Embodiment 3
[0043] In this example, the stimulus-sensitive multifunctional polyisocyanate III with active groups was synthesized.
[0044] Take 4.4 g of N,N'-di-tert-butoxycarbonyl-L-cystine and dissolve it in 50 ml of dichloromethane, add 4.33 g of N,N'-dicyclohexylcarbodiimide and 4.05 g of 1 -Hydroxybenzotriazole, then add 1.38 g of propargylamine, naturally return to room temperature and react for 24 hours. After the reaction was complete, the precipitate was removed by filtration, the product was evaporated to dryness and dissolved in ethyl acetate, washed three times with saturated sodium bicarbonate solution, once each with saturated sodium chloride solution and distilled water, and dried overnight with anhydrous magnesium sulfate. After suction filtration, the filtrate was concentrated under reduced pressure and recrystallized from ethyl acetate. The dried crystals were dissolved in 20 ml of dichloromethane, 20 ml of trifluoroacetic acid was added under ice-cooling, stirred at ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com