Catalyst, its preparation method and its application in methanol and diol synthesis
A technology of catalysts and compounds, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., to achieve the effects of improving stability, increasing rate, and improving catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
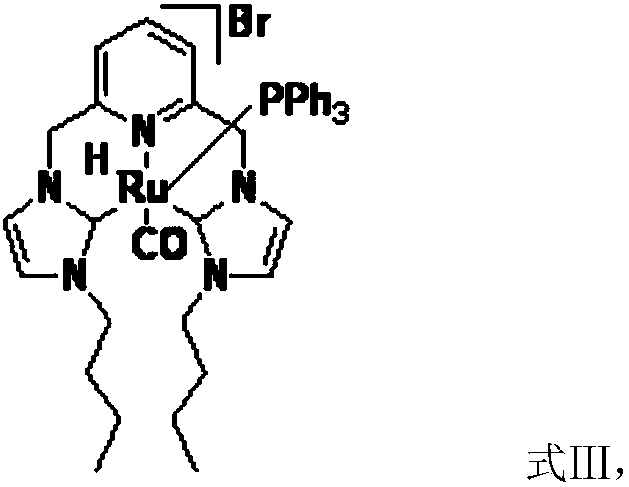
[0031] Another aspect of the present invention provides a method for preparing a catalyst, comprising: preparation of silver carbene complex: nitrogen heterocyclic carbene ligand, Ag 2 O and the first inert solvent are mixed, carry out the first coordination reaction under light-shielding condition, obtain silver carbene complex; The preparation of ruthenium carbene complex catalyst; Silver carbene complex, RuHCl (CO) (PPh 3 ) 3 Mix with the second inert solvent, and carry out the second coordination reaction under the condition of avoiding light to obtain the ruthenium carbene complex catalyst; wherein, the first inert solvent and the second inert solvent are the same or different.
[0032] The present invention adopts functional groups with better stability to water and oxygen as ligands, which is beneficial to improve the stability of the catalyst, thereby solving the problem that the existing catalysts can only be used under relatively severe conditions. At the same time,...
Embodiment 1
[0046] The preparation of bisbutyl silver carbene complex: under the protection of nitrogen, dibutyl imidazolium carbene ligand (0.26g, 0.50mmol), Ag 2 O (0.14g, 0.60mmol) and 30mL anhydrous CH 2 Cl 2 Mix in 100 mL in a two-necked flask, and react in the dark for 24 hours at room temperature. After the reaction, filter to remove unreacted Ag 2 0, the filtrate is spin-dried to obtain dibutylcarbene ligand (0.32g of pale yellow solid, 95% yield), and the performance parameters are as follows: 1 HNMR (400MHz, DMSO): δ=7.79(t, J=8.0Hz, 1H), 7.54(s, 2H), 7.50(s, 2H), 7.21(d, J=8.0Hz, 2H), 5.39(s , 4H), 4.06(t, J=8Hz, 4H), 1.68-1.76(m, 4H), 1.18-1.26(m, 4H), 0.86(t, J=8Hz, 6H); 13 CNMR (100 MHz, DMSO): δ = 180.16, 155.59, 138.78, 122.64, 121.59, 55.34, 50.78, 32.91, 19.07, 13.44.
[0047] Preparation of dibutyl ruthenium carbene complex: under the protection of nitrogen, take successively the above-mentioned dibutyl silver carbene complex (0.73g, 1mmol), RuHCl(CO)(PPh3) 3 (0.95...
Embodiment 2
[0050] Preparation of bisvinylimidazole silver carbene complex: under nitrogen protection, take bisvinylimidazole carbene ligand (0.23g, 0.50mmol), Ag 2 O (0.14g, 0.60mmol) and 30mL anhydrous CH 2 Cl 2 In a 100mL two-necked flask, react in the dark at room temperature for 24h. After the reaction, filter to remove unreacted Ag 2 0, the filtrate is spin-dried to obtain the bisvinylimidazole ruthenium carbene ligand (brown yellow solid 0.26g, productive rate 93%), and the performance parameters are as follows: 1 HNMR (400MHz, DMSO): δ=7.98(d, J=4.0Hz, 2H), 7.90(t, J=8.0Hz, 1H), 7.72(d, J=4.0Hz, 2H), 7.43(d, J =8.0Hz, 2H), 7.31-7.37(m, 2H), 5.72-5.76(m, 2H), 5.53(s, 4H), 5.11-5.14(m, 2H); 13 CNMR (100 MHz, DMSO): δ = 182.70, 155.48, 138.85, 133.97, 123.83, 122.16, 117.57, 103.92, 55.37.
[0051] Preparation of the bisvinylimidazole ruthenium carbene complex Preparation of the silver carbene complex: under nitrogen protection, successively take the above-prepared silver carben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com