Method for detecting afatinib-dimaleate-related substances through high performance liquid chromatography
A high-performance liquid chromatography and afatinib maleate technology, which is applied in the field of high-performance liquid chromatography for detecting afatinib maleate related substances, can solve the problem that the product quality cannot be effectively controlled, and the process impurity degradation cannot be completely separated. problem, to achieve the effect of good accuracy, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: System suitability testing
[0044] Instrument: Shimadzu LC-20A high performance liquid chromatograph and its workstation VWD (or PDA) detector;
[0045] Chromatographic column: WatersXBridgeC184.6×250mm, 3.5μm;
[0046] Mobile phase A: 0.002mol / L dipotassium hydrogen phosphate buffer (use 2mol / L sodium hydroxide to adjust the pH to 10.0);
[0047] Mobile phase B: acetonitrile;
[0048] Detection wavelength: 254nm;
[0049] Flow rate: 0.5mL / min;
[0050] Injection volume: 10μL;
[0051] Column temperature: 40°C;
[0052] Gradient elution table: see Table 1;
[0054] Take an appropriate amount of afatinib maleate (approximately equivalent to 25mg of afatinib), put it in a 50ml measuring bottle, measure the appropriate amount of impurity I, SM1, CL-SM1, M, D stock solution, dissolve and dilute with methanol Made to contain maleic acid, afatinib, impurities I, SM1, CL-SM1, M, and D at concentrations of 0.24mg / ml, 0.5mg / ml, 5ug...
Embodiment 2
[0057] Example 2: Detection of afatinib and its impurity mixed solution
[0058] Instrument: Shimadzu LC-20A high performance liquid chromatograph and its workstation VWD (or PDA) detector
[0059] Chromatographic column: WatersXBridgeC184.6×250mm, 3.5μm;
[0060] Mobile phase A: 0.002mol / L dipotassium hydrogen phosphate buffer (use 2mol / L sodium hydroxide to adjust the pH to 10.0);
[0061] Mobile phase B: acetonitrile;
[0062] Detection wavelength: 254nm;
[0063] Flow rate: 0.5mL / min;
[0064] Injection volume: 10μL;
[0065] Column temperature: 40°C;
[0066] Gradient elution table: see Table 1;
[0068] Measure the appropriate amount of afatinib maleate, impurity A, B, E, F, G, H, J, L, M, SM1, CL-SM1, and I stock solutions, dissolve and dilute with methanol to make A mixed solution of about 2.5ug / ml of Fatinib, about 5ug / ml of impurity I, and about 0.75ug / ml of other impurity monomers is used as a mixed solution of Afatinib and its im...
Embodiment 3
[0078] Embodiment 3: detection of afatinib bulk drug
[0079] Instrument: Shimadzu LC-20A high performance liquid chromatograph and its workstation VWD (or PDA) detector;
[0080] Chromatographic column: WatersXBridgeC184.6×250mm, 3.5μm;
[0081] Mobile phase A: 0.002mol / L dipotassium hydrogen phosphate buffer (use 2mol / L sodium hydroxide to adjust the pH to 10.0);
[0082] Mobile phase B: acetonitrile;
[0083] Detection wavelength: 254nm;
[0084] Flow rate: 0.5mL / min;
[0085] Injection volume: 10μL;
[0086] Column temperature: 40°C;
[0087] Gradient elution table: see Table 1;
[0089] Take afatinib maleate, add diluent to make a solution containing about 0.5 mg / mL of afatinib.
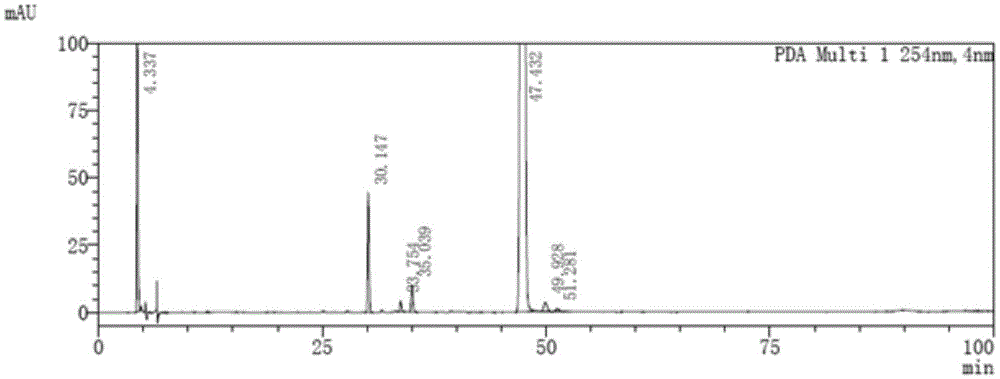
[0090] Get the maleic acid afatinib solution, measure according to the chromatographic conditions of this embodiment, record the chromatogram, the results are shown in Figure 4 .
[0091] Figure 4 The chromatographic peak whose retention time is 47.635min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com