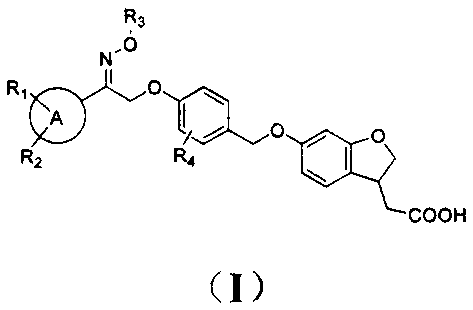
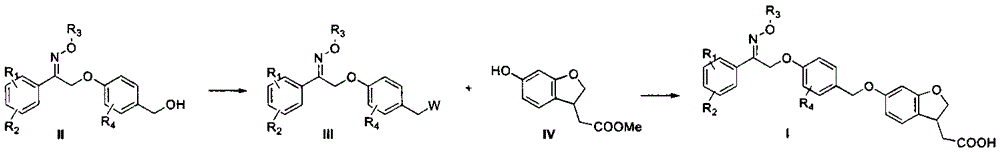
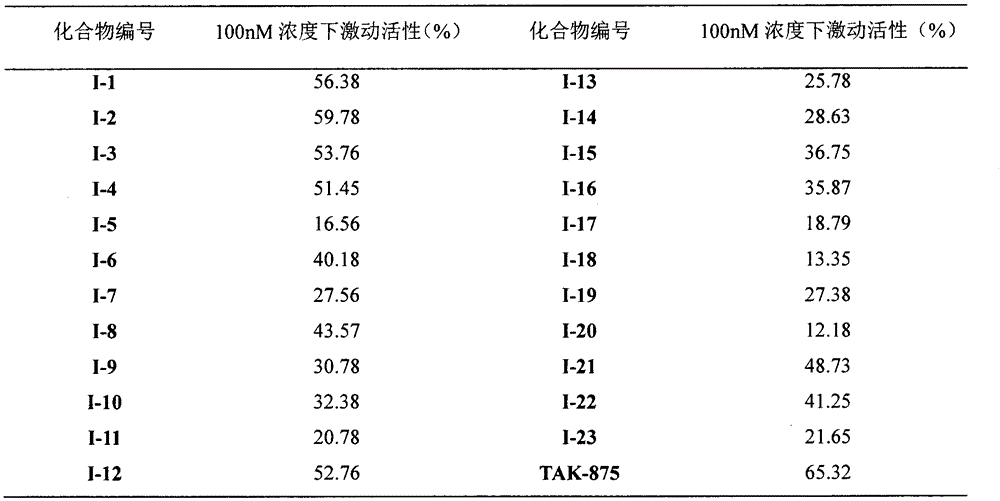
Novel oxime ether derivative and preparation method thereof and application of derivative by serving as drug
A compound, alkoxy technology, applied in the field of preparation of drugs for the treatment of diabetes and metabolic syndrome, can solve problems such as side effects, weight gain, edema side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] (6-Hydroxy-1-benzofuran-3-yl)methyl acetate
[0094]
[0095] Ethyl 4-chloroacetoacetate (4.25ml, 31.43mmol) was dissolved in 20ml of concentrated sulfuric acid at 0°C, and the formed pale yellow viscous solution was cooled to about -5°C in an ice bath, and resorcinol ( 3.15g, 28.57mmol), the internal temperature was controlled below 0°C, the addition was completed, stirred at room temperature for 2h, the reaction solution was poured into 50ml of ice water, a white solid precipitated, suction filtered, washed with water (5ml×2) filter cake, and dried to obtain rice White solid 5.6g, crude product yield 82%.
[0096] Take the above crude product (2g, 9.50mmol) in a 200ml single-necked bottle, add 1N NaOH solution (100ml), the solution immediately becomes thick yellow, the above solution is placed in an oil bath and heated to reflux for 2h, after the reaction is complete, cool to room temperature, and Adjust the pH to 2-3 with sulfuric acid, extract the resulting solu...
Embodiment 2
[0099] (6-Hydroxy-2,3-dihydro-1-benzofuran-3-yl)methyl acetate (IV)
[0100]
[0101] The raw material ester (2g, 9.71mmol) was dissolved in methanol, and a catalytic amount of palladium-carbon 0.2g was added, replaced with hydrogen three times, and stirred at room temperature for 24 hours by introducing hydrogen gas. The solvent was evaporated from the filtrate under reduced pressure to obtain 1.93 g of off-white powdery solid, with a yield of 95%.
[0102] 1 HNMR (300MHz, CDCl 3 )δ: 6.97 (d, J = 8.71Hz, 1H, ArH), 6.31-6.34 (m, 2H, ArH), 4.82 (brs, 1H, ArOH), 4.75 (t, J = 9.10Hz, 1H, -OCH 2 ), 4.26, 4.24 (dd, J=5.72, 9.10Hz, 1H, -OCH 2 ), 3.74-3.84 (m, 1H, ArCH), 3.72 (s, 3H, -OCH 3 ), 2.74, 2.69 (dd, J=5.72, 16.41Hz, 1H, -COCH 2 ), 2.55, 2.50 (dd, J=9.11, 16.41Hz, 1H, -COCH 2 ).
Embodiment 3
[0104] 2-(6-(4-(2-methoxyimino-2-phenethoxy)benzyloxy)-2,3-dihydro-1-benzofuran-3-yl)acetic acid (I- 1)
[0105]
[0106] Step 1: Dissolve 2-bromoacetophenone (0.7g, 3.52mmol) in 15ml of DMSO, add methoxylamine hydrochloride (0.4g, 5.28mmol), after the addition is complete, stir at room temperature for 5h, add 150ml of water to dilute, Extracted with ethyl acetate (30ml×3), combined the organic phases, washed with saturated brine (15ml×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled off the solvent under reduced pressure to obtain 0.8g of a yellow-brown oil, which was used directly react in the next step.
[0107] Second step: get above-mentioned oily product (0.8g, 3.52mmol) and dissolve in 25ml acetonitrile, add potassium carbonate (1.46g, 10.56mmol) successively, 4-hydroxybenzyl alcohol (0.44g, 3.52mmol), the above-mentioned mixture is heated for 65 Reaction at ℃ for 8 hours, suction filtration, the filtrate was evaporated to remove th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com