The preparation method of neratinib intermediate
A neratinib and intermediate technology, applied in the field of organic synthesis, can solve the problems of low yield and high reaction temperature, and achieve the effects of reducing reaction temperature, avoiding high-temperature reaction and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
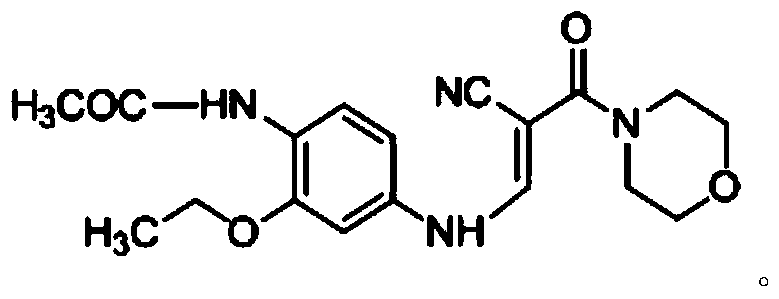
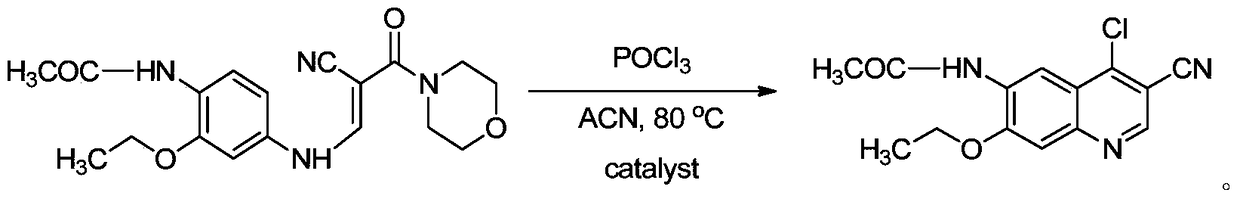
[0018] Example 1 Preparation of 4-chloro-3-cyano-7-ethoxy-6-acetylquinoline
[0019] Add compound A (7.17g, 20mmol), catalyst triethylamine (286mg, 2mmol), acetonitrile (100mL) into a 500mL two-neck flask, and a magnetic stirring bar. Then install the reflux condenser, the constant pressure dropping funnel, and then add POCl to the constant pressure dropping funnel 3 (7.4mL, 80mmol). A mixed solution of acetonitrile (44mL) was added dropwise in 15 minutes. Under stirring, slowly drop the mixed solution into the reactant system, and the reaction solution gradually becomes a transparent solution. The reaction solution was heated to 65° C., and reacted under this condition for 5 hours. The reaction solution was detected with a TLC plate, and it was found that the raw material disappeared completely, and a compound with a lower polarity than the raw material was generated, and the compound appeared blue under a 254 nm ultraviolet lamp. At this time, the heating reaction was sto...
Embodiment 2
[0020] Example 2 Preparation of 4-chloro-3-cyano-7-ethoxy-6-acetylquinoline
[0021] Add compound A (7.17g, 20mmol), catalyst N,N-lutidine (122mg, 1.0mmol), propionitrile (100mL) and a magnetic stirrer into a 500mL two-necked flask. Then install the upper reflux condenser and constant pressure dropping funnel. Add POCl to the constant pressure dropping funnel 3 (9.2mL, 100mmol). A mixed solution of propionitrile (44mL) was added dropwise in 15 minutes. Under stirring, slowly drop the mixed solution into the reactant system, and the reaction solution gradually becomes a transparent solution. The reaction solution was heated to 70° C., and reacted under this condition for 3 hours. The reaction solution was detected with a TLC plate, and it was found that the raw material disappeared completely, and a compound with a lower polarity than the raw material was generated, and the compound appeared blue under a 254 nm ultraviolet lamp. At this time, the heating reaction was stoppe...
Embodiment 3
[0022] Example 3 Preparation of 4-chloro-3-cyano-7-ethoxyl-6-acetylquinoline
[0023] Add compound A (7.17g, 20mmol), catalyst triethylenediamine (200mg, 1.8mmol), ethyl acetate (100mL) and a magnetic stirring bar into a 500mL two-necked flask. Then install the reflux condenser and the constant pressure dropping funnel, and drop it in 15 minutes. Add POCl to the constant pressure dropping funnel 3 (8.3mL, 90mmol) and ethyl acetate (44mL) mixed solution. Under stirring, slowly drop the mixed solution into the reactant system, and the reaction solution gradually becomes a transparent solution. The reaction solution was heated to 75° C., and reacted under this condition for 2 hours. The reaction solution was detected with a TLC plate, and it was found that the raw material disappeared completely, and a compound with a lower polarity than the raw material was generated, and the compound appeared blue under a 254 nm ultraviolet lamp. At this time, the heating reaction was stopp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com