Fluorine-containing double-tail hydrophobically associating polymer and preparation thereof
A hydrophobic association and polymer technology, applied in drilling compositions, chemical instruments and methods, etc., can solve the problems of weakened synergy of oil-displacing agents and poor oil-displacing effect, and achieve low surface tension, enhanced The effect of stickiness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
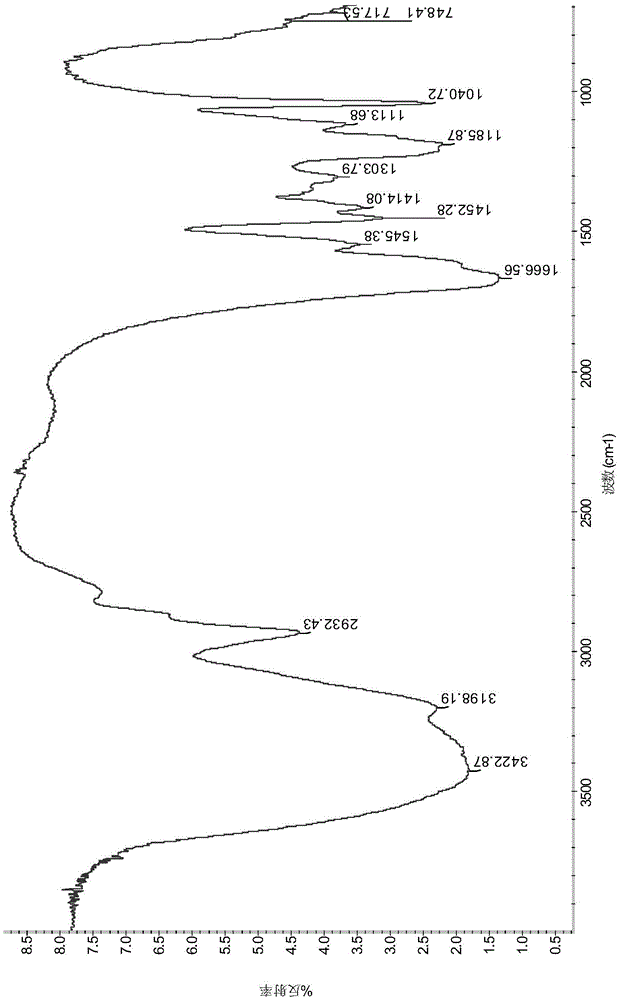
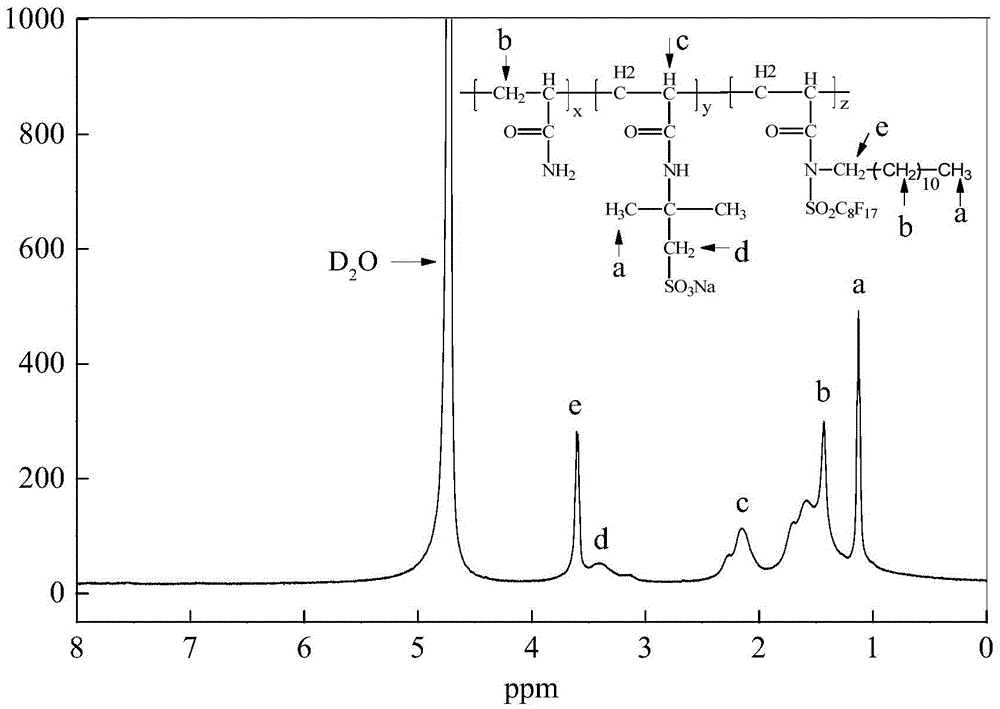
Image
Examples
preparation example Construction
[0022] Preferably, the preparation method of the fluorine-containing double tail hydrophobic association polymer comprises the following steps:
[0023] Step S1, after mixing 1-2 mole fractions of long-chain alkylamine with 1-2 mole fractions of triethylamine and an organic solvent, add 1 mole fraction of perfluorooctanesulfonyl Fluorine, then raise the temperature to 30-60°C, continue the reaction for 4-10h, the reaction mixture is washed, dried, and distilled under reduced pressure to obtain the product N-alkyl perfluorooctane sulfonamide.
[0024] Further preferably, the organic solvent in step S1 is one or more of toluene, isopropanol, isopropyl ether, acetone or xylene.
[0025] Further preferably, the long-chain alkylamine in step S1 is one of n-hexylamine, n-octylamine, dodecylamine, tetradecylamine, cetylamine, and octadecylamine.
[0026] Further preferably, the reaction mixture in step S1 is washed successively with water, 0.5% hydrochloric acid aqueous solution, sa...
Embodiment 1
[0035] The first step: Synthesis of fluorine-containing double tail polymerizable macromonomer AMPD
[0036] S1, Synthesis of N-dodecyl perfluorooctane sulfonamide
[0037] Add 0.15 mol of dodecylamine, 0.2 mol of triethylamine and 100 mL of isopropyl ether into a 250 mL three-necked flask, mix well and slowly add 0.12 mol of perfluorooctanesulfonyl fluoride dropwise under stirring conditions. Then the temperature was raised to 50°C, and the reaction was continued for 5h. The reaction mixture was washed successively with water, 0.5% hydrochloric acid aqueous solution, and saturated brine, then dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain the product N-alkylperfluorooctanesulfonamide.
[0038] S2, Synthesis of Macromonomer AMPD
[0039] Add 0.1 mol N-dodecyl perfluorooctane sulfonamide to the three-neck flask, and add dichloromethane to form a 40% solution. Stir vigorously and blow nitrogen gas, control the temperature at 3°C, slowly...
Embodiment 2
[0048] The first step: Synthesis of fluorine-containing double tail polymerizable macromonomer AMPD
[0049] S1, Synthesis of N-dodecyl perfluorooctane sulfonamide
[0050] Add 0.15 mol of dodecylamine, 0.18 mol of triethylamine and 120 mL of acetone into a 250 mL three-necked flask, mix well and slowly add 0.12 mol of perfluorooctanesulfonyl fluoride dropwise under stirring conditions. Then the temperature was raised to 45°C, and the reaction was continued for 5h. The reaction mixture was washed successively with water, 0.5% hydrochloric acid aqueous solution, and saturated brine, then dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain the product N-dodecylperfluorooctanesulfonamide.
[0051] S2, Synthesis of Macromonomer AMPD
[0052] Add 0.15mol N-dodecyl perfluorooctane sulfonamide to the three-necked flask, and add dichloromethane to form a 40% solution. Stir vigorously and blow nitrogen gas, control the temperature at 4°C, slowly ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com