A synthetic process of L-menthol
A synthesis process, menthol technology, applied in the field of L-menthol synthesis process, can solve the problems of harsh reaction conditions, low efficiency, high reaction temperature and pressure, etc., to achieve improved esterification yield, easy operation, high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
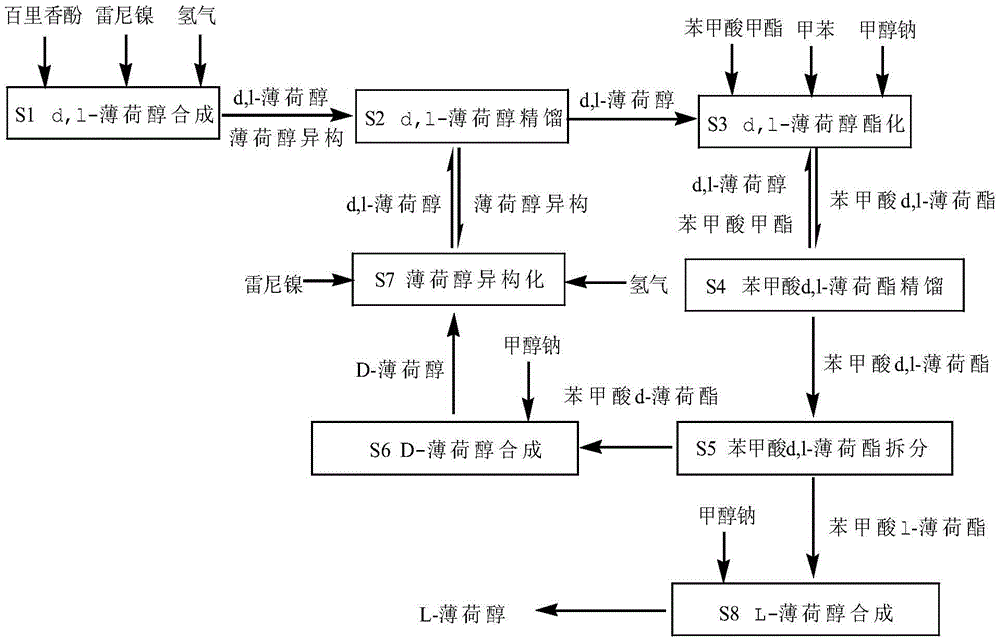
[0077] S1. Synthesis of d,l-menthol
[0078] In a 5L hydrogenation kettle, 1923g of thymol and 57.7g of Raney nickel were added, and hydrogen gas was introduced. The molar ratio of hydrogen to thymol was 1:4, and the pressure of the kettle was 2MPa. The reaction temperature is 190°C, and the hydrogenation reaction generates 20.37% of d,l-neomenthol, 65.58% of d,l-menthol, 1.84% of d,l-neoisomenthol, and 10.21% of d,l-isomenthol.
[0079] S2, d,l-menthol rectification
[0080] The above reaction was rectified under reduced pressure to obtain d,l-menthol 1311.6g, l-isomenthol, d,l-neomenthol, d,l-neo-menthol isomeric mixture 648.4g, this step The yield is 98% of theoretical.
[0081] S3, d,l-menthol esterification
[0082] Add 5109g of methyl benzoate, 1953.5g of d,l-menthol, 30% sodium methoxide and toluene into a 20L esterification reactor, wherein the molar weight of methyl benzoate is 3 times that of d,l-menthol, 30% Sodium methoxide 4508g, molar weight is d, 2 times of ...
Embodiment 2
[0094] S1. Synthesis of d,l-menthol
[0095] In a 5L hydrogenation kettle, 1923g of thymol and 38.5g of Raney nickel were added, and hydrogen was introduced. The molar ratio of hydrogen to thymol was 1:4, and the pressure of the kettle was 2MPa. The reaction temperature was 170°C, and the hydrogenation reaction produced 22.37% of d,l-neomenthol, 62.26% of d,l-menthol, 1.22% of d,l-neoisomenthol, and 11.15% of d,l-isomenthol.
[0096] S2, d,l-menthol rectification
[0097] The above reaction was rectified under reduced pressure to obtain d,l-menthol 1245.2g, l-isomenthol, d,l-neomenthol, d,l-neoisomenthol isomeric mixture 694.8g, this step The yield is 97% of theoretical.
[0098] S3, d,l-menthol esterification:
[0099] Add 3306g of methyl benzoate, 1919.1g of d,l-menthol, 30% sodium methoxide and toluene into a 20L esterification reactor, wherein the molar weight of methyl benzoate is 3 times that of d,l-menthol, 30% Sodium methoxide 4428.8g, molar weight is 2 times of d, l...
Embodiment 3
[0111] S1. Synthesis of d,l-menthol
[0112] In a 5L hydrogenation kettle, 1923g of thymol and 19.2g of Raney nickel were added, hydrogen was introduced, the molar ratio of hydrogen to thymol was 1:4, and the pressure of the kettle was 1MPa. The reaction temperature was 160°C, and the hydrogenation reaction produced 21.60% of d,l-neomenthol, 58.36% of d,l-menthol, 1.39% of d,l-neoisomenthol, and 13.65% of d,l-isomenthol.
[0113] S2, d,l-menthol rectification
[0114] The above reaction was rectified under reduced pressure to obtain 1167.2 g of d,l-menthol, 732.7.4 g of isomeric mixtures of d,l-neomenthol, d,l-neomenthol, and 1,167.2 g of l-isomenthol, respectively. The step yield is 95% of the theoretical yield.
[0115] S3, d,l-menthol esterification
[0116] Add 3262g of methyl benzoate, 1870.6.5g of d, l-menthol, 30% sodium methoxide and toluene in a 20L esterification reactor, wherein the molar weight of methyl benzoate is twice that of d, l-menthol, 30 % sodium metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com