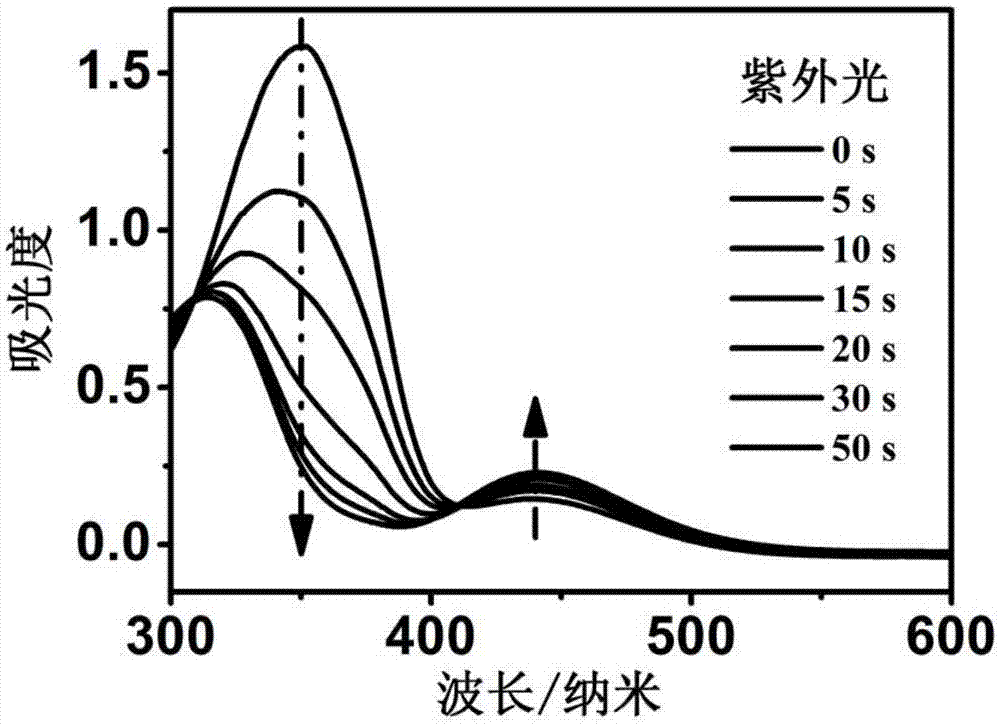
Preparation method of multi-responsiveazobenzene functionalized polymer
A multi-response, azobenzene technology, applied in the field of organic chemistry, can solve the problems of photoresponsive group shedding, polymer molecular structure and performance without light reversible changes, etc., achieving low reaction temperature and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
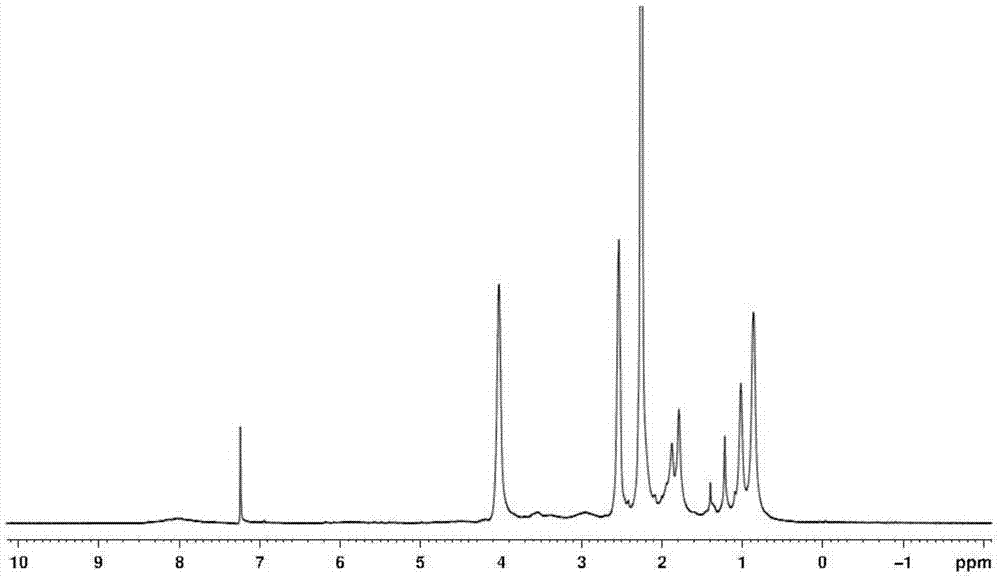
[0025] Preparation of polydimethylaminoethyl (meth)acrylate: add 8 ml of anhydrous tetrahydrofuran to a 10 ml dry schlenk bottle, and add complexing agents to the schlenk bottle in sequence under nitrogen protection 0.144 g of 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA), 8.334 g of monomer dimethylaminoethyl methacrylate (DMAEMA), after 15 minutes, add 0.076 g of catalyst Cuprous bromide (CuBr), then stop filling the nitrogen gas, and stopper the bottle mouth with a rubber stopper. After three cycles of liquid nitrogen freezing-suction-thawing, after the air in the bottle is exhausted, the schlenk bottle is slowly heated to 40 degrees Celsius, and after the temperature is stable, the initiator α-bromoisobutyrate ethyl Ester (EBIB) 0.065 ml, reacted at 40 degrees Celsius for 24 hours. After the reaction is over, quench the schlenk bottle with liquid nitrogen to terminate the reaction, and add a large amount of CH 2 Cl 2 Dilute the product, then use a neutral alumin...
Embodiment 2
[0027] Preparation of m-trifluorotoluene azophenol: 3.22 g of m-trifluorotoluidine, 6 ml of concentrated HCl (38%) and 12 ml of deionized water were added to a 250 ml three-neck flask. Add 1.394 g NaNO to a 100 ml flask 2 and 7 ml of deionized water, stirred to dissolve. Under the condition of 0-5 degrees Celsius, slowly drop the sodium nitrite solution into the m-trifluorotoluidine / hydrochloric acid solution and stir for 2 hours. After the addition is completed, stir for another 2 hours to make the reaction complete. When the starch potassium iodide test paper turns blue, filter the product, and cool the filtrate (yellow) to obtain a diazonium salt solution.
[0028] Add 1.88 grams of phenol, 1.2 grams of NaOH and 10 milliliters of deionized water into a 250-ml beaker, and dissolve 10 milliliters of 1.88 grams of NaOH at 0-5 2 CO 3Add the aqueous solution dropwise into the phenol / sodium hydroxide solution. After stirring completely, add the above-mentioned diazonium salt d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com