Method for detecting Afatinib and relevant substances thereof
A detection method and technology of related substances, which are applied in the detection of afatinib cis-isomers, afatinib and its related substances, can solve the problems of poor practicability and applicability, lack of universal adaptability, Heptafluorobutyric acid is expensive, etc., to achieve the effect of good peak shape, strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
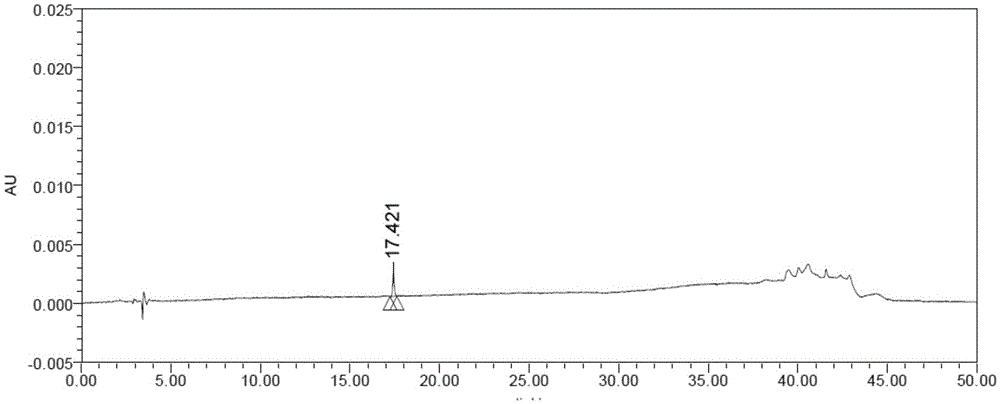
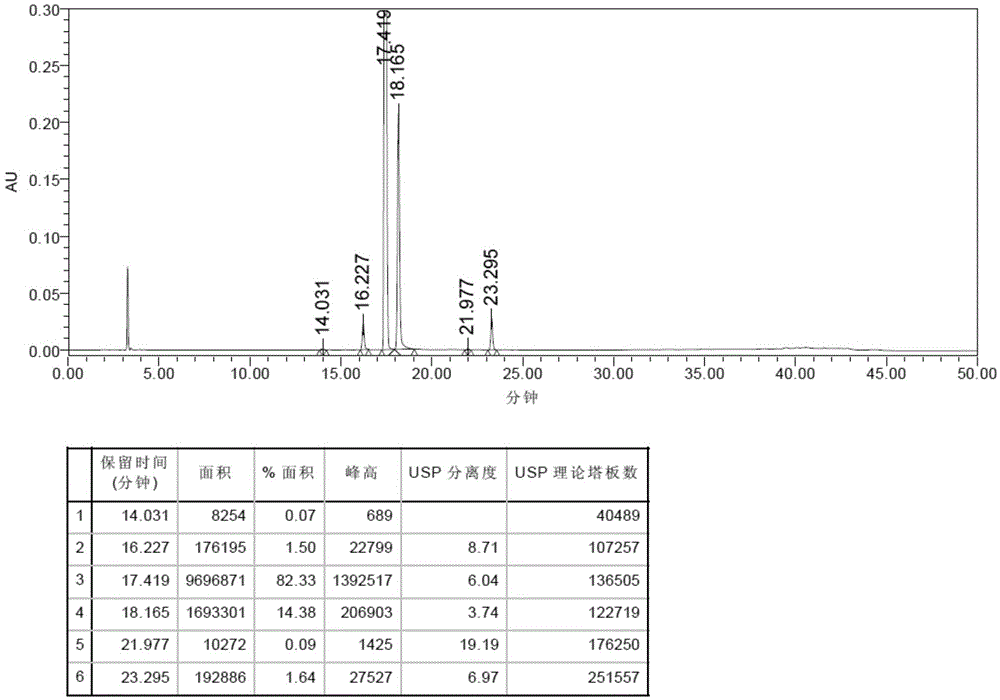
Image
Examples
Embodiment 1
[0037] (1) Detection conditions
[0038] Instrument: waters2695 high performance liquid chromatography, 2996PDA detector, detection wavelength: 260nm;
[0039] Chromatographic column: Phenomenex C18 (250mm×4.6mm, 5μm) chromatographic column;
[0040] Diluent: 30% acetonitrile;
[0041] Mobile phase: an aqueous solution of 0.2% formic acid+0.1% trifluoroacetic acid (take 2ml of formic acid and 1ml of trifluoroacetic acid solution and mix them in 1000ml of water) as mobile phase A, and acetonitrile as mobile phase B;
[0042] Flow rate: 1.0ml / min;
[0043] Column temperature: 40°C;
[0044] Injection volume: 10uL;
[0045] Carry out linear gradient elution according to Table 1.
[0046] Table 1
[0047]
[0048] (2) Detection steps
[0049] Preparation of resolution solution: Accurately weigh the cis-isomer (RC1), afatinib maleate, RC3 and RC4 and mix them in a certain proportion to form a resolution solution.
[0050] Preparation of reference solution: Take an ap...
Embodiment 2
[0055] According to the similar detection method of Example 1, wherein the detection wavelength is replaced by 255nm. The chromatogram of its reference substance is shown in Figure 5 , The chromatogram of the test product see Figure 6 .
Embodiment 3
[0057] According to the similar detection method of Example 1, wherein the detection wavelength is replaced by 265nm. The chromatogram of its reference substance is shown in Figure 7 , The chromatogram of the test product see Figure 8 .
[0058] Methodology Validation Test Results
[0059] According to the "Chinese Pharmacopoeia" 2015 edition guideline 9101 drug quality standard analysis method validation guideline part, the method validation test was carried out, and the chromatographic conditions for durability investigation included flow rate ± 0.1mL / min, column temperature ± 5 ℃, mobile phase Ratio ±2%, wavelength ±2nm. The results are shown in Table 2.
[0060] Table 2
[0061]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com