Amphiphilic block polymer having folate-targeted pH-reduction dual-response and antineoplastic activity and preparation as well as application thereof
An anti-tumor activity, dual response technology, applied in the field of biomedical applications and polymer chemistry, can solve problems such as cell damage, and achieve the effect of promoting inhibition, reducing damage, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] (1) compound Preparation: Weigh 3.08g of bis(2-hydroxyethyl) disulfide and dissolve it in 30mL of dichloromethane, add 4g of triethylamine as an acid-binding agent and stir for 20 minutes, slowly drop 1.08 g of methacryloyl chloride was added, and the stirring reaction was continued for 24 hours. After the reaction was complete, the reaction solution was washed three times with 100 mmol / l pH of phosphate buffer and distilled water of 8.0, the organic layer was dried over anhydrous sodium sulfate, and then the solvent was rotary evaporated, and the crude product was purified by silica gel chromatography to obtain a white 1.62 g solid. is the compound , and the yield was 73%.
[0075] 1 HNMR (400MHz, CDCl 3 ,δ,ppm):6.13(s,1H,CH H =C(CH 3 )CO-),5.59(s,1H,C H H=C(CH 3 )CO-),4.41(t,J=6.6Hz,2H,-COOC H 2 CH 2 SSCH 2 CH 2 OH),3.87(t,J=6.5Hz,2H,-COOCH 2 CH2 SSCH 2 C H 2 OH),2.96(t,J=6.7Hz,2H,-COOCH 2 C H 2 SSCH 2 CH 2 OH),2.87(t,J=5.8Hz,2H,-COOCH 2 CH 2...
Embodiment 2
[0091] (1) compound , , , , , and compound The preparation: with embodiment 1.
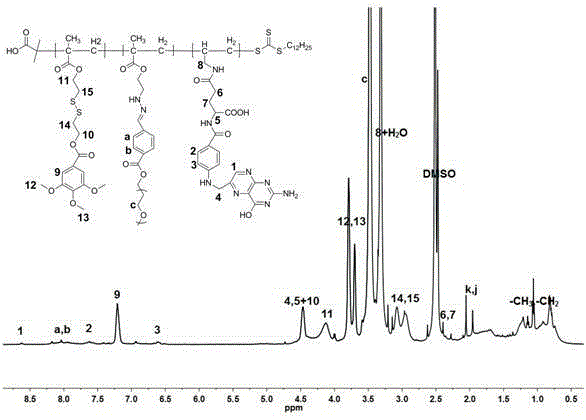
[0092] (2) Target compound Preparation: take 0.11g compound (Mn=1.18×10 4 g / mol), 24mg of folic acid monomer and 26mg of AIBN were dissolved in 3ml of DMSO in a Schlenk flask, filled with nitrogen in an ice bath for 30 minutes, then put the Schlenk flask into a constant temperature oil bath, and reacted at 60°C for 24 hours. The reaction solution was precipitated with ether to obtain 0.104g light yellow powder compound , and the yield was 58%. Mn:1.42×10 4 .
[0093] 1 HNMR (400MHz, DMSO-d6, δ, ppm): 8.65 (–C H –of FA heterocyclic), 8.12-7.95 (4H, m, phenyl group of acid labile PEG-macroRAFT), 7.66 (–CH 2 NHC 6 H 2 h 2 CONHCH(COOH)CH 2 CH 2 CO–ofFA), 6.65 (–CH 2 NHC 6 h 2 H 2 CONHCH(COOH)CH 2 CH 2 CO–ofFA), 4.46 (–C H 2 NHC 6 h 4 CONHCH(COOH)CH 2 CH 2 CO–ofFA), 4.41 (2H,t,-COOCH 2 CH 2SSCH 2 C H 2 COOC 6 h 2 (OCH 3 ) 3 of MAOHD-TMOBA), 4.33 (–CH...
PUM
| Property | Measurement | Unit |
|---|---|---|
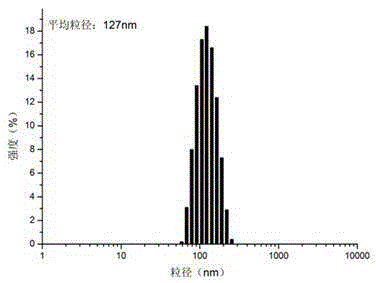
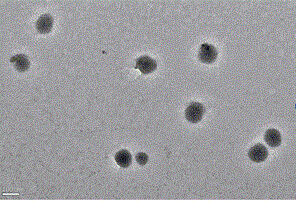
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com