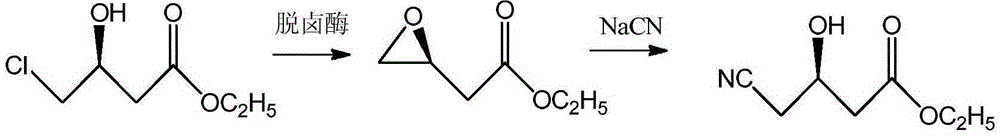
Process of using resin-immobilized halohydrin dehalogenase to catalytically synthesize (R)-4-cyan-3-hydroxy ethyl butyrate
A technology of ethyl hydroxybutyrate and halohydrin dehalogenase, which is applied in the field of medicine and chemical industry, can solve the problems of low purity of the final product, enzyme residue, and purification effects, etc., and achieve improved substrate conversion rate, mild reaction conditions, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of technology that resin immobilizes halohydrin dehalogenase catalytic synthesis (R)-4-cyano-3-hydroxybutyric acid ethyl ester comprises the following steps:
[0033] 1) Soak the AB8 resin with distilled water to remove impurities, first soak the AB8 resin with 3-5wt% NaOH solution 3-5 times the volume of the AB8 resin for at least 1 hour, then rinse it with distilled water until it is neutral, and then wash it with 2-3 times the volume of AB8 resin Soak the AB8 resin in 3-5wt% HCl solution of the resin volume for at least 0.5h, and finally rinse it with distilled water until it is neutral, and alternately soak it for 2-4 times to get pre-treated AB8 resin, and use more than 2 times the volume of AB8 resin at 4°C Soak in distilled water for later use;
[0034] 2) Prepare a 48U / mL halohydrin dehalogenase solution and place it at 4°C for use; prepare a 0.1 mol / L disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a pH of 7 for use;
[0035...
Embodiment 2-9
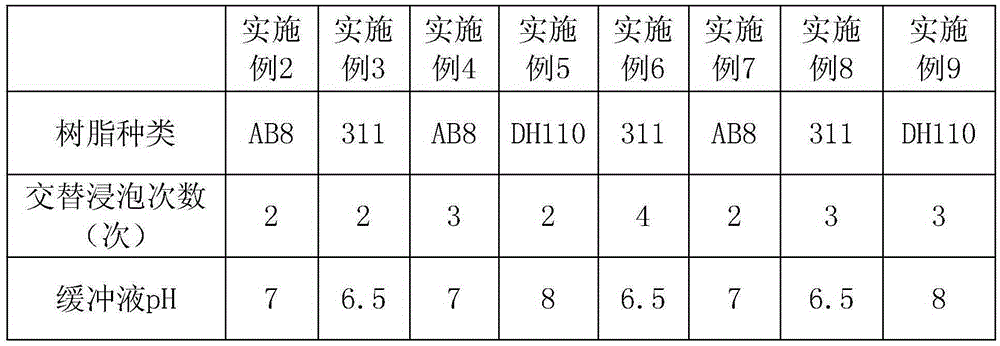
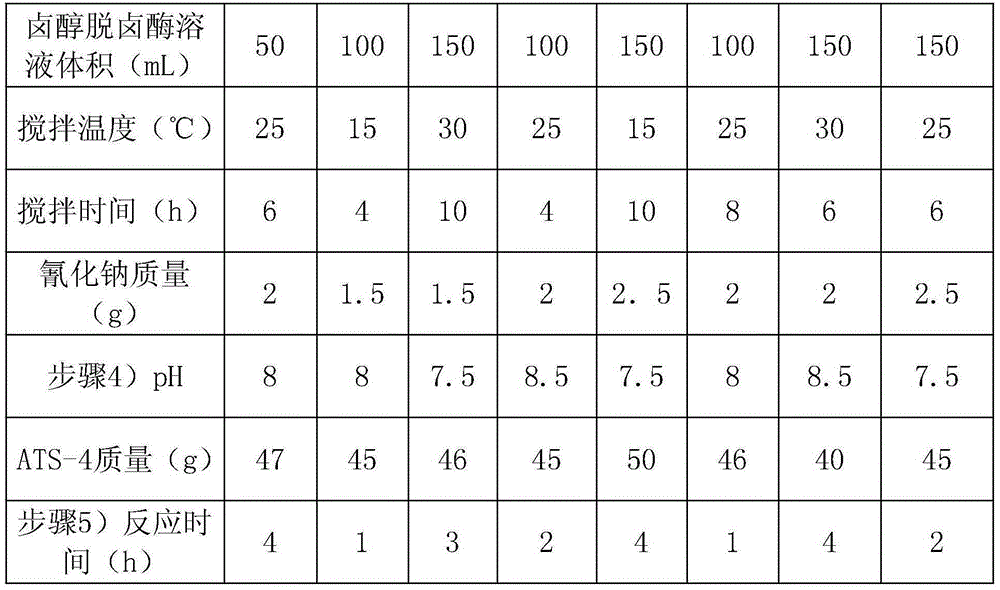
[0039] Each embodiment is basically the same as embodiment 1, the difference lies in table 1.
[0040] Table 1
[0041]
[0042]
[0043] Table 2 shows the immobilization rate of halohydrin dehalogenase and its ATS-5 production rate after immobilization in each embodiment.
[0044] Table 2
[0045]
[0046] Note: where "the first time" is the generation rate of directly using the obtained adsorption resin immobilized halohydrin dehalogenase to catalyze the synthesis of (R)-4-cyano-3-hydroxybutyrate ethyl ester, "the second time " is the rate of formation of (R)-4-cyano-3-hydroxybutyrate ethyl ester in the recovered adsorption resin immobilized halohydrin dehalogenase after the completion of the first synthesis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com