Novel intermediate for synthesizing topirastat and its preparation method
A technology of topirax and intermediates, applied in the field of drug synthesis, can solve the problems of long time, low total yield, unfavorable industrialized large-scale production, etc., and achieve the effects of increased yield, low cost, and easy control of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
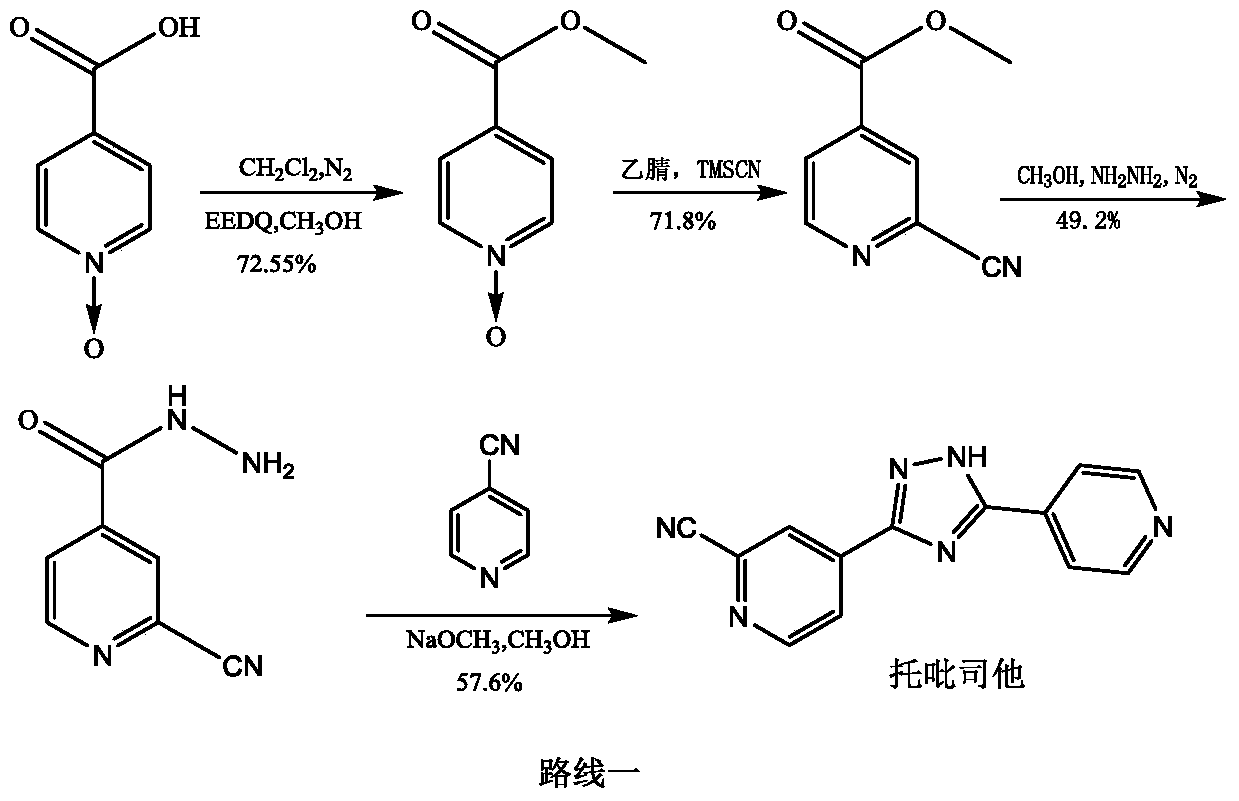
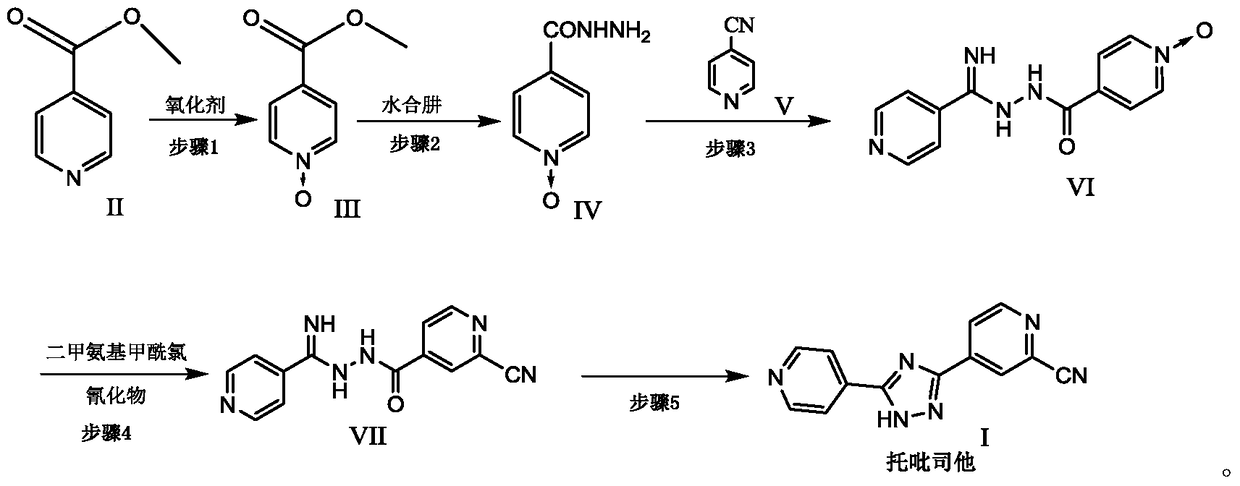
[0032] The preparation of embodiment one methyl isonicotinate N-oxide compound:
[0033] 1) Put methyl isonicotinate (20g, 145.8mmol) in the reaction flask, add 120ml of acetic acid, stir, then add 30% hydrogen peroxide (16.5g, 145.6mmol) into the reaction flask, heat and stir at 70°C, after 3h , add 30% hydrogen peroxide (11.6g, 102.3mmol), continue to heat and stir, after the reaction is monitored by TLC, concentrate, add 50ml of water, extract with 500ml of dichloromethane, concentrate after drying the organic phase, stir and wash with n-hexane, and filter with suction , a pale yellow crystalline solid was obtained, dried in vacuo at 50° C. to obtain 21.26 g, yield: 95.2%. [M+H] + = 154.03. 1 H NMR (400MHz, CDCl 3 )δ: 3.86-3.91 (s, 3H), 7.78-7.87 (d, 2H), 8.13-8.22 (d, 2H)
[0034] 2) Put methyl isonicotinate (20g, 145.8mmol) in the reaction flask, add 120ml of ethanol, stir, then add 30% hydrogen peroxide (16.5g, 145.6mmol) into the reaction flask, heat and stir at 70°...
Embodiment 2
[0035] The preparation of embodiment diisoniazid N-oxide compound:
[0036] 1) Put methyl isonicotinate N-oxide (20g, 130.6mmol) in the reaction flask, add 200ml of methanol, stir until completely dissolved, then add 85% hydrazine hydrate (14.5g, 246.2mmol) dropwise, nitrogen protection , heated at 60°C for 2h, cooled to room temperature, added 50ml of isopropyl ether and stirred, filtered with suction to obtain an off-white solid, dried in vacuum at 45°C, dry weight 19.3g, yield: 96.5%. [M-H] + = 152.04. 1 H NMR (400 MHz, DMSO) δ: 4.50-4.70 (s, 2H), 7.75-7.82 (d, 2H), 8.26-8.33 (d, 2H), 9.98-10.10 (s, 1H).
[0037] 2) Add methyl isonicotinate N-oxide (2.0g, 13.1mmol) and 20ml of dichloromethane into the reaction flask, stir until completely dissolved, add 85% hydrazine hydrate (1.5g, 25.5mmol) dropwise, and finish , under nitrogen protection, after heating at 40°C for 2h, a large amount of yellow solid precipitated out, stopped heating, cooled to room temperature, and filt...
Embodiment 3
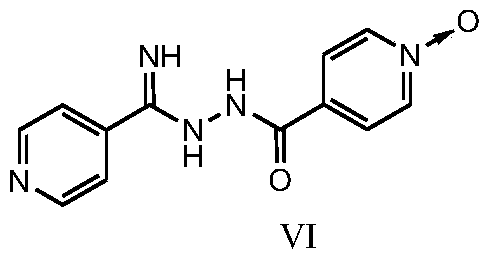
[0038] Example 3 Preparation of 4-(2-(imino(pyridin-4-yl)methyl)hydrazinecarbonyl)pyridine N-oxide:
[0039] 1) Put 4-cyanopyridine (11.0g, 106.0mmol) in a reaction flask, add 150ml of methanol, stir until completely dissolved, add 55mg of sodium methoxide, protect with nitrogen, heat at 40°C, react for 2 hours, then add isoniazid N-oxide (15.0g, 98.0mmol), reacted for 3h, cooled to room temperature, added isopropyl ether (90ml) into the bottle, stirred, and filtered with suction to obtain a yellow powdery solid, vacuum-dried at 50°C, dry weight 24.4g , Yield: 96.8%. [M+H] + = 258.10. 1 H NMR (400MHz, DMSO) δ: 6.87-7.13(br, 2H), 7.75-7.83(d, 2H), 7.88-7.93(d, 2H), 8.28-8.36(d, 2H), 8.62-8.71(d , 2H), 9.98-10.70 (br, 1H).
[0040] 2) Put 4-cyanopyridine (11.0g, 106.0mmol) in the reaction flask, add 150ml of ethanol, stir until completely dissolved, add sodium ethoxide (60mg), protect with nitrogen, heat at 40°C, react for 2 hours, add iso Niacinazine N-oxide (15.0g, 98.0mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com