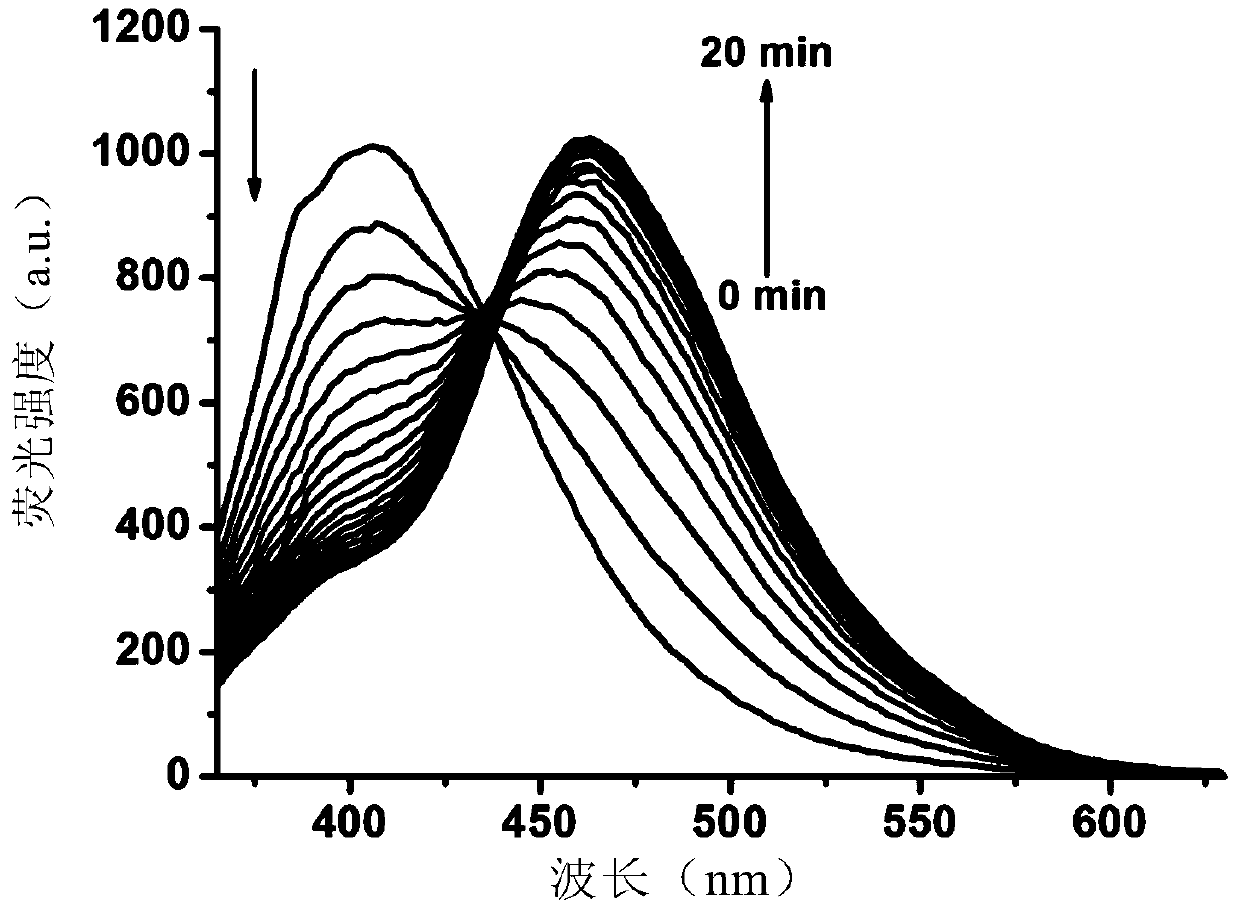
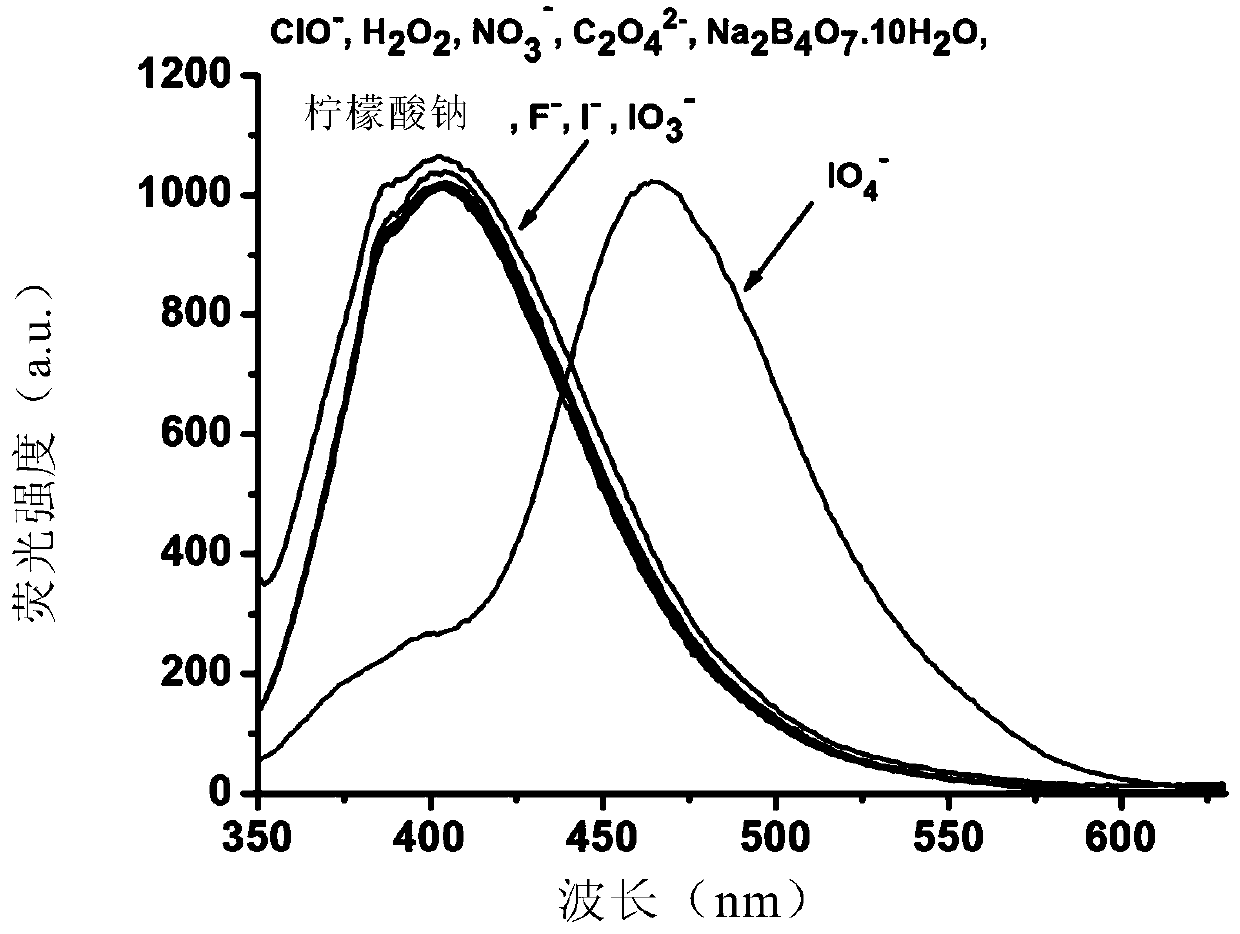
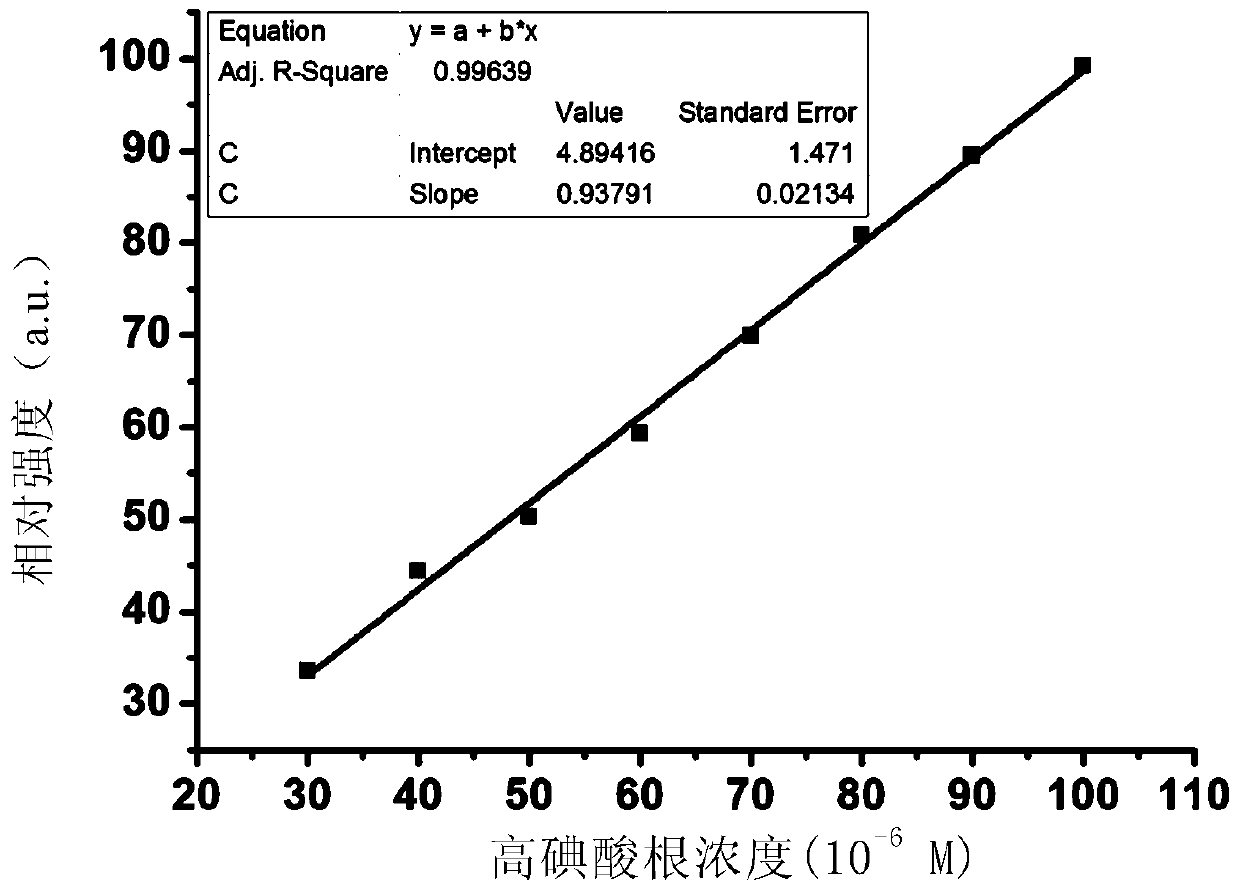
A highly selective ratiometric fluorescent probe for the detection of periodate
A technology of acetic acid solution and compound, which is applied in the field of anion detection, can solve the problems of repeatability difference in periodate detection experiment results, large time, instrument and labor costs, and influence on the quantitative detection of periodate, etc., to achieve solid fluorescence signal stability, Advanced internal calibration function, good selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0074]
[0075] HMBT or its derivatives can be prepared by the method described by Z.Xu et al. (Chemical Communications, 48(88), 10871-10873(2012)).
[0076] It should be understood that although the above preparation scheme only provides the preparation of compound PDS-2, those skilled in the art can prepare the compounds of formula II and formula I of the present invention by changing the groups of the reactants. For example, different trihydric alcohols with different carbon chain lengths can be used to prepare compounds A, B or C with corresponding carbon chain lengths.
[0077] The present invention also includes the compound of formula I of the present invention as a fluorescent probe in the detection of periodate in a chemical system, the fluorescent imaging test of periodate in biological living cells, the construction of a high-throughput hydrolase test platform, and the detection of test strips. Apps in development.
[0078] In a specific embodiment, the detectio...
Embodiment 1
[0084] Embodiment 1: the synthetic route of probe PDS-2
[0085] (1) Preparation of 2-(2-benzothiazolyl)-6-methoxyphenol (HMBT)
[0086]
[0087]Refer to literature (Z.Xu et al., Chemical Communications, 48(88), 10871-10873(2012)).
[0088] (2) Preparation of 2-(2,2-dimethyl-1,3-4-dioxolanyl)ethanol (A)
[0089]
[0090] Weigh 2-4g of 1,2,4-butanetriol and dissolve it in acetone, then add 300-400mg of p-toluenesulfonic acid monohydrate (TsOH), then stir the above reaction mixture at room temperature for two days, Finally, 0.7 mL of triethylamine was added to terminate the reaction, and evaporated to dryness with a rotary evaporator to obtain a crude product, which was then separated by column chromatography on silica gel to obtain a colorless oily product.
[0091] 1 H NMR (400MHz, CDCl 3 )δ4.19(br,1H),4.01(br,1H),3.69(br,2H),3.51(br,1H),1.74(br,2H),1.34(s,3H),1.29(s,3H ); 13 C NMR (100MHz, CDCl 3 )δ108.85,74.53,69.38,59.89,35.79,26.80,25.60; MS(ESI)m / z 147.09[M+H...
Embodiment 2
[0112] Embodiment 2: the influence of pH value on the fluorescence intensity of PDS-2 in the aqueous solution
[0113] Perform pH titration in water. In the pure water system, add 1 mM probe DMSO mother solution to obtain 10 μM probe aqueous solution, use hydrochloric acid and sodium hydroxide aqueous solution to adjust the probe aqueous solution at different pH, and measure the corresponding fluorescence spectrum respectively. A pH titration curve was obtained by plotting the pH and the fluorescence intensity at the maximum emission wavelength (406nm and 462nm). In order to determine the optimum pH condition of the test system.
[0114] The result is as Figure 7 shown. There was no change in the fluorescence intensity of PDS-2 in the pH range of 6-10. Therefore, PBS buffer solution with a pH of 7.4 and containing 1% DMSO was selected as the test medium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com