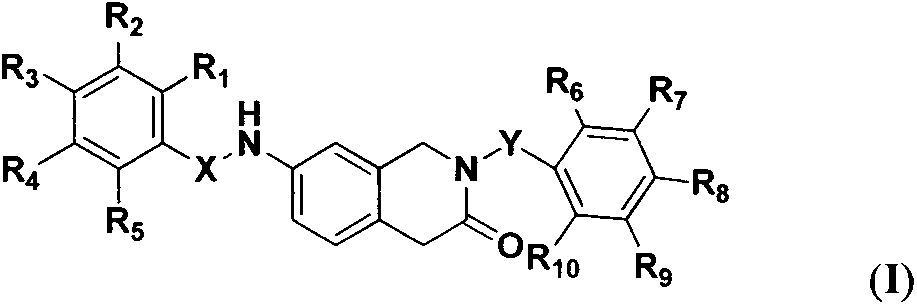
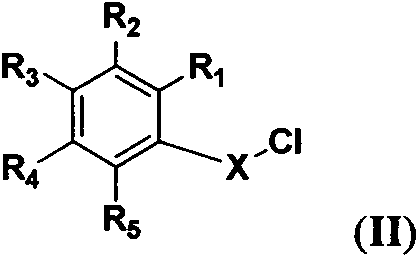
7-amino-1,4-dihydroisoquinoline-3(2H)-one derivative, synthetic method and application thereof
A technology of dihydroisoquinoline and its derivatives, which can be applied in drug combinations, nervous system diseases, organic chemistry, etc., and can solve the problems of low compound activity, increased side effects, undisclosed acetylcholinesterase inhibitory activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 2-N-benzoyl-7-N-benzoylamino-1,4-dihydroisoquinolin-3(2H)-one (I 1 ) preparation
[0030] (1) Preparation of 2-indanone (IV)
[0031]Add 140ml of 88% formic acid and 28ml of 30% hydrogen peroxide in a 250ml three-necked bottle equipped with a thermometer and a constant pressure dropping funnel, and control the temperature of the water bath at 35-40°C. Accurately measure 26ml of 90% indene into the bottle through the dropping funnel and slowly add it dropwise, stirring while dropping. After 2 hours, the dropping is completed, wash the dropping funnel with 10ml of formic acid, and continue stirring at 35°C for 20 minutes. Then it was stirred for 7 hours at 25°C. After the reaction, excess formic acid in the reaction system was evaporated by a rotary evaporator (35mmHg / 35-40°C). After the rotary evaporation, the residual liquid will be cooled and a white solid will be precipitated. Before the next step, it can be slightly heated to liquefy it, which is convenient for ad...
Embodiment 2
[0046] 2-N-(3'-chlorobenzoyl)-7-N-benzoylamino-1,4-dihydroisoquinolin-3(2H)-one (I 2 ) preparation
[0047] At room temperature, take 70mgIX 1 Dissolve it with 10ml of dioxane, and add 0.1ml of triethylamine as an acid-binding agent. Dissolve 0.1ml of m-chlorobenzoyl chloride in 10ml of dioxane, and add it dropwise into the reaction system through the dropping funnel. press I 1 Preparation method, 40mg viscous yellow product was obtained with a yield of 39.5%.
[0048] 1 HNMR (500MHz, CDCl 3 )δ7.93(d, J=4.6Hz, 3H, Ar-H), 7.86-7.68(s, 1H, CO-NH-Ar), 7.63-7.24(m, 9H, Ar-H), 4.97(d , J=10.3Hz, 2H, Ar-CH 2 -N), 3.79(d, J=14.5Hz, 2H, Ar-CH 2 -CO)
Embodiment 3
[0050] 2-N-(3'-methylbenzoyl)-7-N-benzoylamino-1,4-dihydroisoquinolin-3(2H)-one (I 3 ) preparation
[0051] At room temperature, take 70mgIX 1 Dissolve it with 10ml of dioxane, and add 0.1ml of triethylamine as an acid-binding agent. Dissolve 0.1ml of m-toluoyl chloride in 10ml of dioxane, and add it dropwise into the reaction system through the dropping funnel. press I 1 Preparation method, 40mg viscous yellow-brown product was obtained with a yield of 39.6%.
[0052] 1 HNMR (500MHz, CDCl 3 )δ7.95-7.84 (m, 2H, Ar-H), 7.79 (s, 1H, CO-NH-Ar), 7.79-7.49 (m, 4H, Ar-H), 7.45-7.29 (m, 4H, Ar-H), 7.16(d, J=7.5Hz, 2H, Ar-H), 4.96(d, J=8.7Hz, 2H, Ar-CH 2 -N), 3.78(d, J=14.1Hz, 2H, Ar-CH 2 -CO), 2.42(s, 3H, Ar-CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com