Slowly released type sirolimus eye-drops preparation and preparation method thereof
A sirolimus ophthalmic, sustained-release technology, applied to non-active ingredient medical preparations, active ingredient-containing medical preparations, pharmaceutical formulations, etc., can solve problems such as poor solubility and stability of sirolimus , to achieve the effect of low incidence of adverse reactions, good intraocular penetration and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
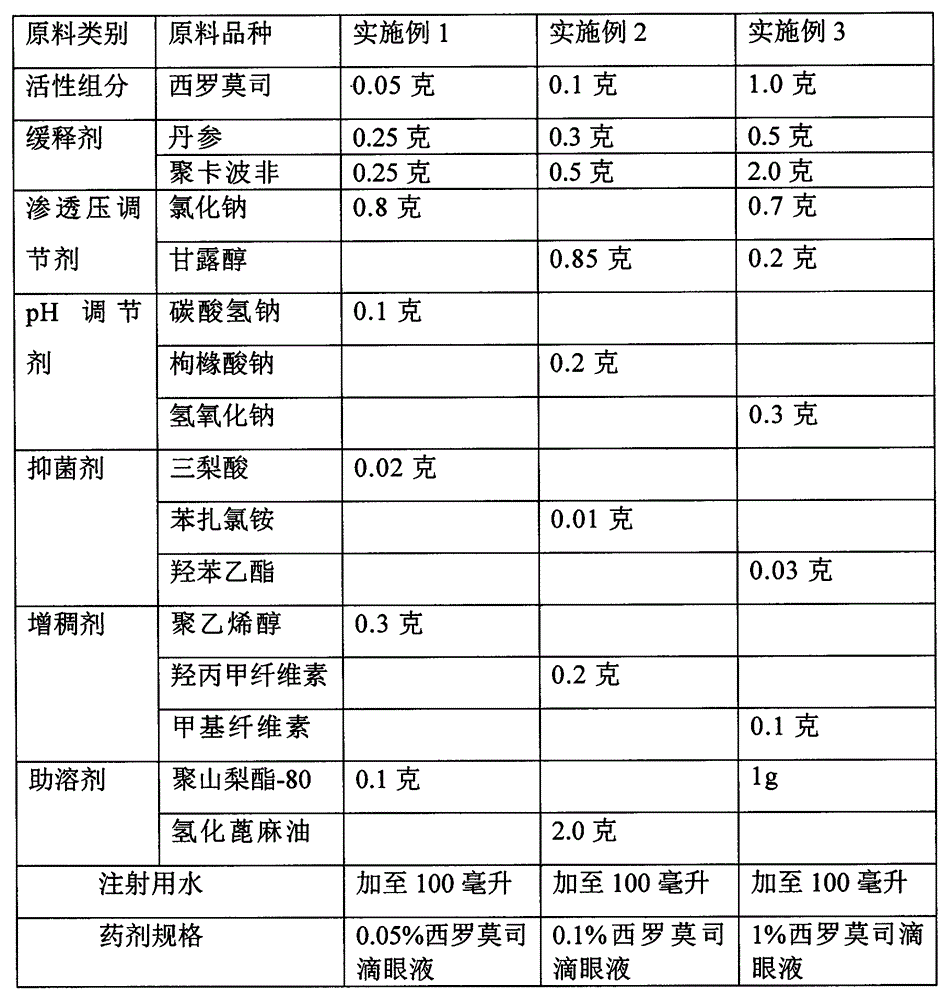
[0021] Embodiment 1-3 prepares sustained-release sirolimus eye drops raw material components and consumption
[0022]
[0023] According to the technical scheme of the present invention, the adjuvant materials that can be selected for the preparation of sirolimus eye drops are not limited to the species listed in the above table, and there are also multiple options, such as bacteriostatic agents, any bacteriostatic agents known in pharmacy can be used , and its dosage is according to the conventional dosage in pharmacy. For example, ① 0.002% ~ 0.005% thimerosal; ② quaternary ammonium salts (including benzalkonium chloride, benzalkonium bromide), dumiphene, spirityl, etc., the effective concentration is 0.002% ~ 0.01%; ③ alcohols, commonly used 0.3 ~ 0.6% chlorobutanol; ④ Parabens, commonly used 0.03-0.06% ethylparaben; ⑤ Acids, such as 0.01-0.08% tricolic acid. The concentrations of the above substances are all volume-weight percentages, that is, every 100 milliliters cont...
Embodiment 4-6
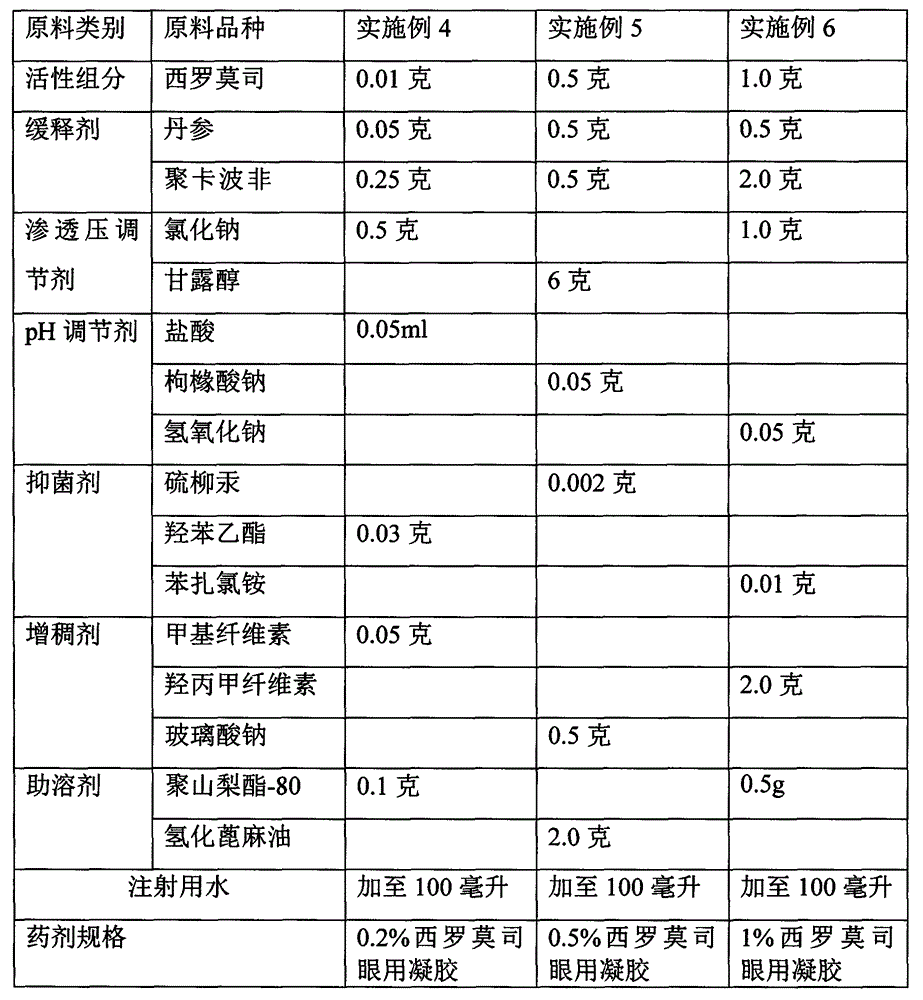
[0025] Example 4-6 Preparation of Sustained Release Sirolimus Ophthalmic Gel Raw Material Components and Consumption
[0026]
[0027] According to the technical scheme of the present invention, the types of auxiliary materials that can be used to prepare sirolimus ophthalmic gel are not limited to the types listed in the above table, and the following multiple choices can also be made: wherein, the type selection and dosage of the bacteriostatic agent are the same as in Example 1 ~3. The dosage ratio of the thickener to sirolimus is 0.5-5.0:1.0.
[0028] The preparation method is as follows: dissolve the whole amount of sirolimus with salvia miltiorrhiza, dissolve the thickener with water for injection, disperse and let it cool, and dissolve the pH regulator and bacteriostat with water for injection, and then add the thickener and western medicine that have been dissolved. Rolimus is obtained by adding water for injection to the required volume and aseptically dispensing....
Embodiment 7-9
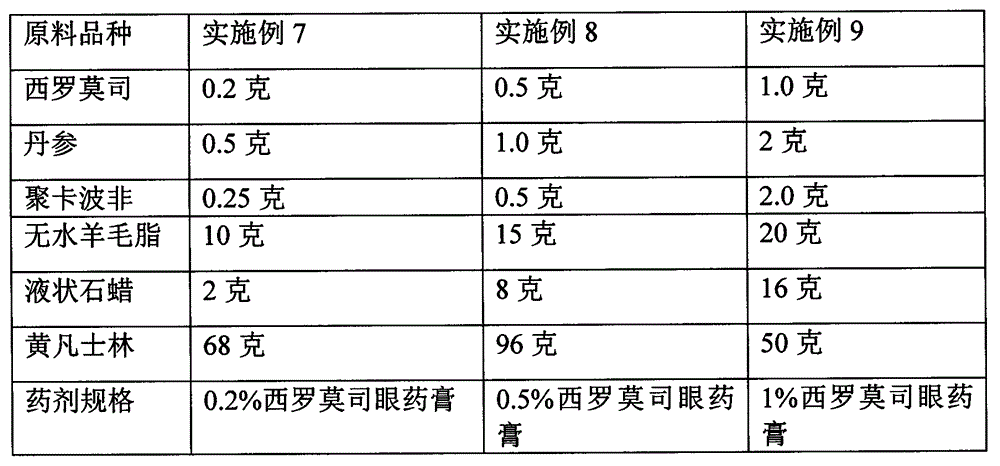
[0029] Embodiment 7-9 Preparation of sustained-release sirolimus ophthalmic ointment raw material components and dosage
[0030]
[0031] Preparation method: dissolve the whole amount of sirolimus with Salvia miltiorrhiza, add an appropriate amount of sterilized and cooled liquid paraffin, grind it into a fine paste, pass through a 200-mesh sieve, and then gradually add sterile and filtered lanolin and yellow petrolatum mixture, Mix well and serve. All preparation equipment and packaging containers used must be sterilized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com