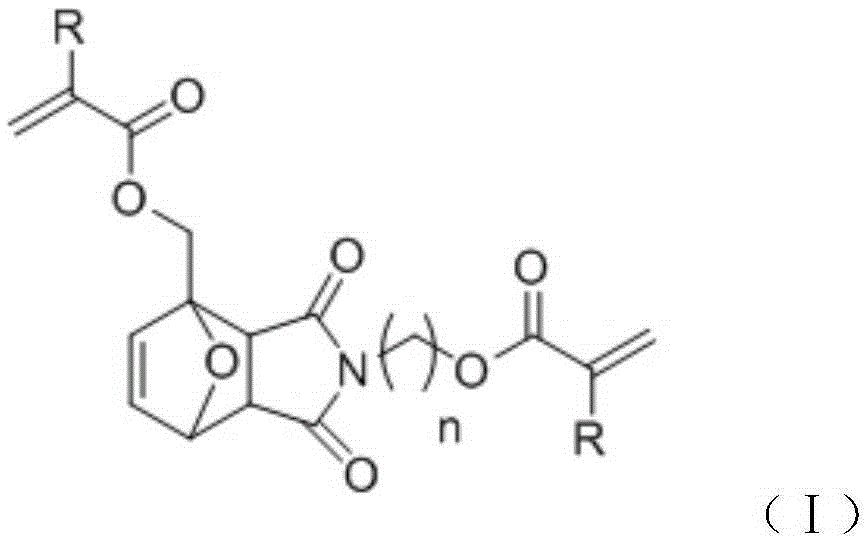
Bi-functionality-degree acrylic ester cross-linking agent and application thereof to 3D printing
An acrylate and acrylate technology, applied in the field of light-curing 3D printing rapid prototyping to prepare polymer materials, can solve problems such as immaturity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0042] The synthesis of embodiment 1 difunctionality methacrylate crosslinking agent
[0043] 1) Preparation of dihydroxy Diels-Alder adducts:
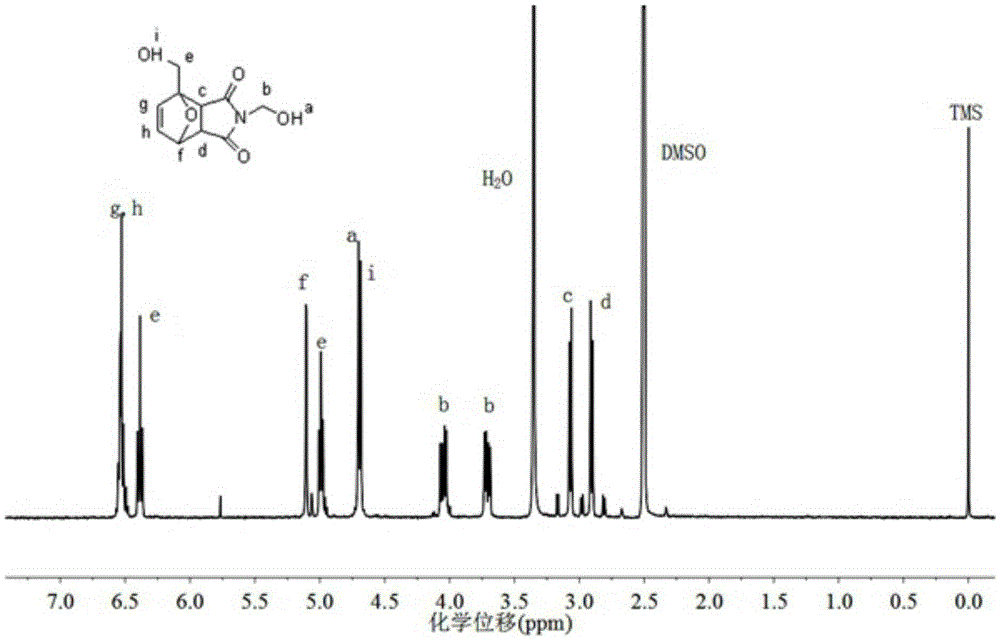
[0044] Under nitrogen protection, 12.7g (0.1mol) N-methylolmaleimide (CAS: 5063-96-7), 19.6g furfuryl alcohol (0.2mol) (CAS: 98-00-0), 200ml of ethyl acetate was added into a 500ml three-neck flask, and under magnetic stirring, the reaction was carried out at reflux temperature (about 77° C.) and protected from light for 24 hours. After the reaction, cool, filter with suction, collect the filter cake, wash with cold ethyl acetate three times, collect the filter cake, and vacuum-dry at 30°C to constant weight to obtain the target product as a white solid powder with a yield of 83%. The structure of the product was confirmed by spectral characterization, and its NMR spectrum is shown in the attached figure 1 .
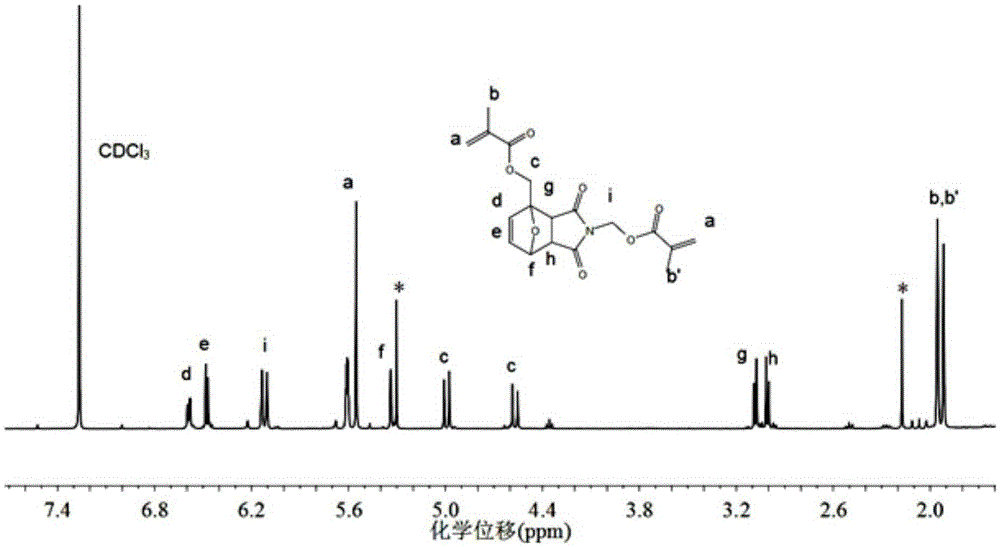
[0045] 2) Preparation of difunctional methacrylate crosslinking agent
[0046] Under nitrogen protection, 22.5g (0.1mol) o...
Embodiment 2 2
[0047] Synthesis of embodiment 2 difunctionality acrylate crosslinking agent
[0048] 1) Preparation of dihydroxy Diels-Alder adducts:
[0049] Under nitrogen protection, 14.1g (0.1mol) N-hydroxyethylmaleimide (CAS: 1585-90-6), 24.5g furfuryl alcohol (0.4mol) (CAS: 98-00-0), 250ml of ethyl acetate was added into a 500ml three-necked flask, and reacted at 60°C in the dark for 48 hours under magnetic stirring. After the reaction, cool, filter with suction, collect the filter cake, wash with cold ethyl acetate three times, collect the filter cake, and vacuum-dry at 30°C to constant weight to obtain the target product as a white solid powder with a yield of 81%. The structure of the product was confirmed by 1H NMR spectrum, and its 1H NMR spectrum was omitted.
[0050] 2) Preparation of difunctional acrylate crosslinking agent
[0051] Under the protection of nitrogen, successively add 23.9g (0.1mol) of the dihydroxy Diels-Alder adduct obtained in the above step (1), 15.8g (0.2...
Embodiment 3 2
[0052] Synthesis of embodiment 3 difunctionality acrylate crosslinking agent
[0053] 1) Preparation of dihydroxy Diels-Alder adducts:
[0054] Under nitrogen protection, 12.7g (0.1mol) N-methylolmaleimide (CAS: 5063-96-7), 9.8g furfuryl alcohol (0.1mol) (CAS: 98-00-0), 300ml of ethyl acetate was added into a 500ml three-neck flask, and under magnetic stirring, the reaction was carried out at reflux temperature (about 77° C.) and protected from light for 48 hours. After the reaction, cool, filter with suction, collect the filter cake, wash with cold ethyl acetate three times, collect the filter cake, and vacuum-dry at 30°C to constant weight to obtain the target product as a white solid powder with a yield of 83%. Spectral characterization confirmed the structure of the product, and its NMR spectrum was omitted.
[0055] 2) Preparation of difunctional acrylate crosslinking agent
[0056] Under nitrogen protection, 22.5g (0.1mol) of the dihydroxy Diels-Alder adduct obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com