Non-precious metal oxygen reduction catalyst and preparing method and application thereof
A non-precious metal, catalyst technology, used in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc. and other problems, to achieve the effect of easy mass production, easy control of feeding amount and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, preparation and performance test of non-precious metal oxygen reduction catalyst
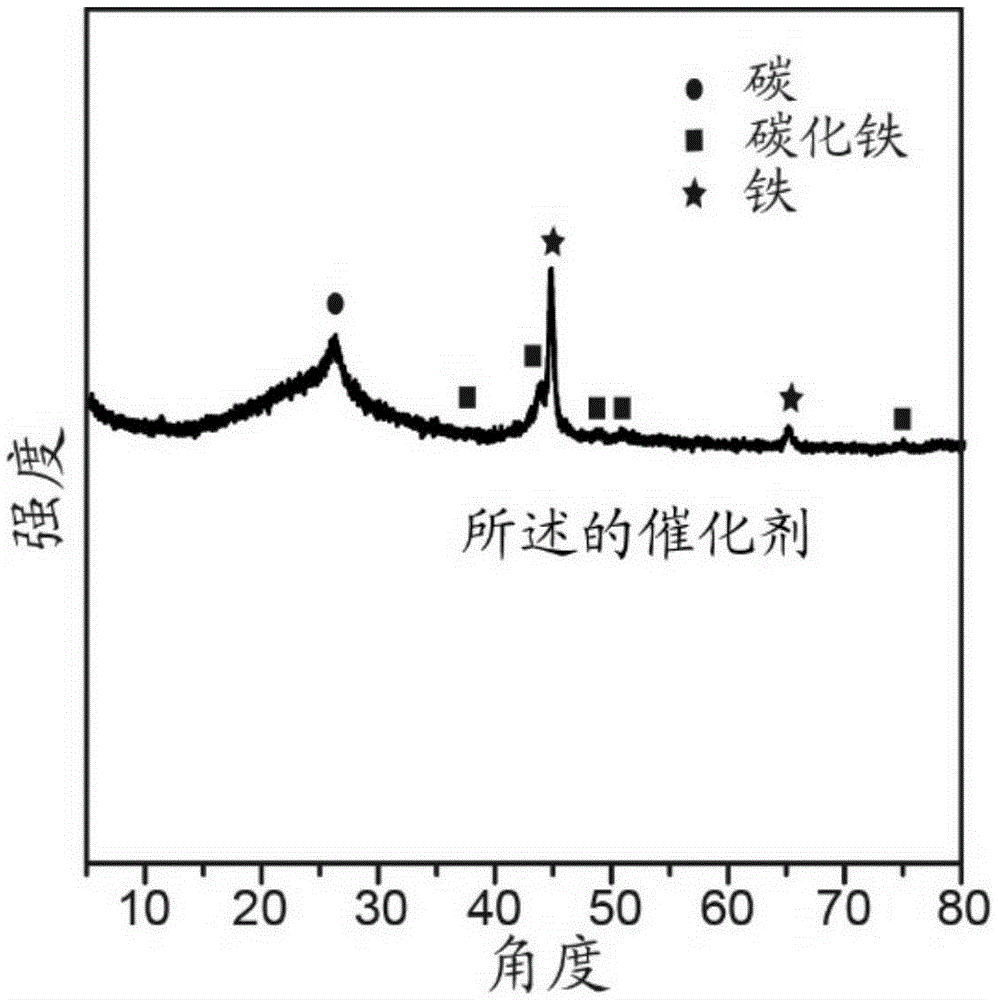
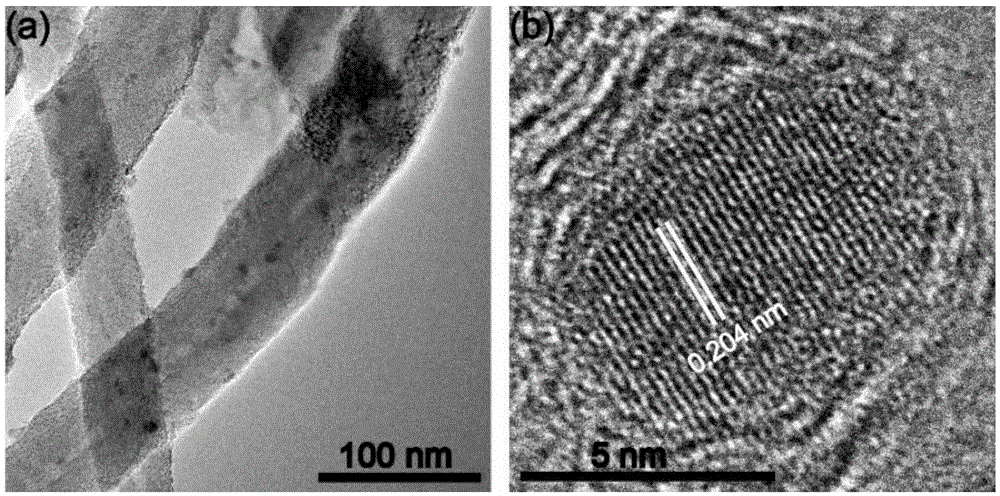
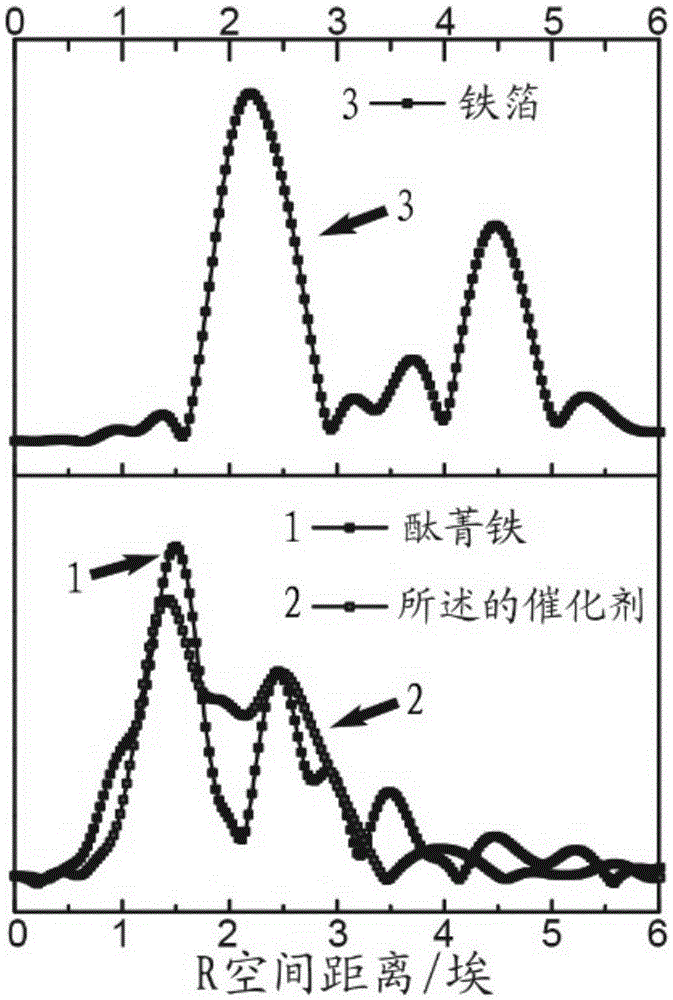
[0033] After ultrasonically dispersing 20 mg of carbon nanotubes (40 to 60 nanometers in diameter and 5 to 15 microns in length) in 10 mL of water for 1 hour, add 800 mg of glucose and 600 mg of ferric nitrate nonahydrate, and transfer them to 25 mL for polymerization after being completely dissolved. In a high-pressure reactor lined with tetrafluoroethylene, then carry out hydrothermal reaction at a temperature of 180°C for 15 hours, wash with water and ethanol several times, and filter with suction to obtain a solid, which is dried overnight at 60°C to obtain a precursor ; After mixing the precursor and melamine at a mass ratio of 1:10, transfer it to a porcelain boat, and put it into a quartz tube of a tube furnace, deair it with argon for half an hour, and then raise the temperature to 900°C. After heat treatment for 2 hours under the protection of argon, a non-noble met...
Embodiment 2
[0041] Embodiment 2, preparation and performance test of non-precious metal oxygen reduction catalyst
[0042] A non-noble metal oxygen reduction catalyst was prepared basically in the same manner as in Example 1, except that the amount of iron salt was changed from 600 mg to 200 mg, and the obtained catalyst contained almost no metallic iron or carbonized carbon coated with graphite carbon. Iron nanoparticles, containing only FeN 4 Coordination structure; the half-wave potential obtained by testing the oxygen reduction curve in 0.1 mole per liter of potassium hydroxide solution is 0.862V.
Embodiment 3
[0043] Embodiment 3, preparation and performance test of non-precious metal oxygen reduction catalyst
[0044] A non-noble metal oxygen reduction catalyst was prepared basically in the same manner as in Example 1, except that the amount of iron salt was changed from 600 mg to 1000 mg, and the catalyst obtained was similar in composition to the catalyst obtained in Example 1, but metal iron or The particle size of the iron carbide nanoparticles increases obviously; the half-wave potential obtained by testing the oxygen reduction curve in 0.1 mole per liter of potassium hydroxide solution is 0.885V.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com