A class of compounds derived from forsythia, its preparation method and its application in the prevention and treatment of Parkinson's disease
A compound and composition technology, applied in the field of medicine, can solve the problems of Parkinson's disease of traditional Chinese medicine Forsythia suspensa and the patent documents of preparation method of monomeric forsythia compound, etc., and achieve the inhibition of neuroinflammatory response and structure of microglia. Novel, inhibitory effect on induced neurotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
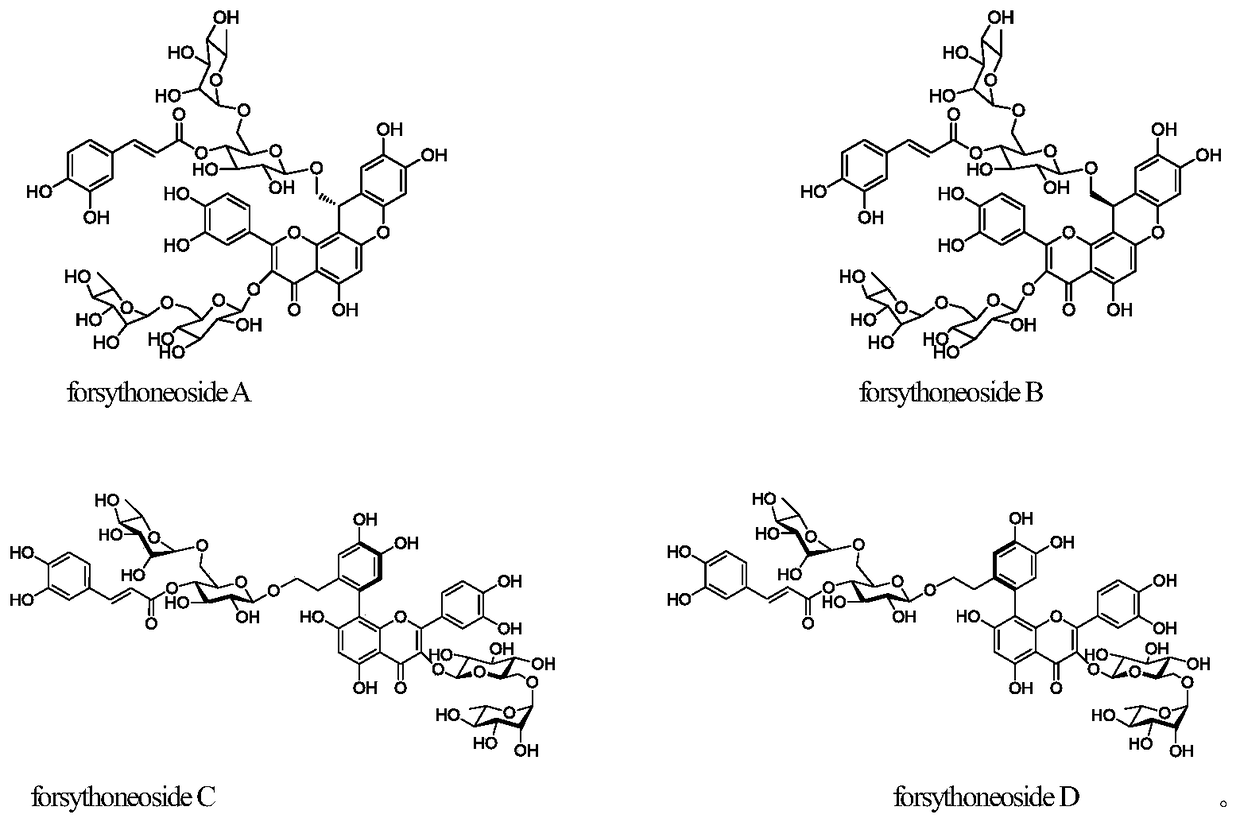
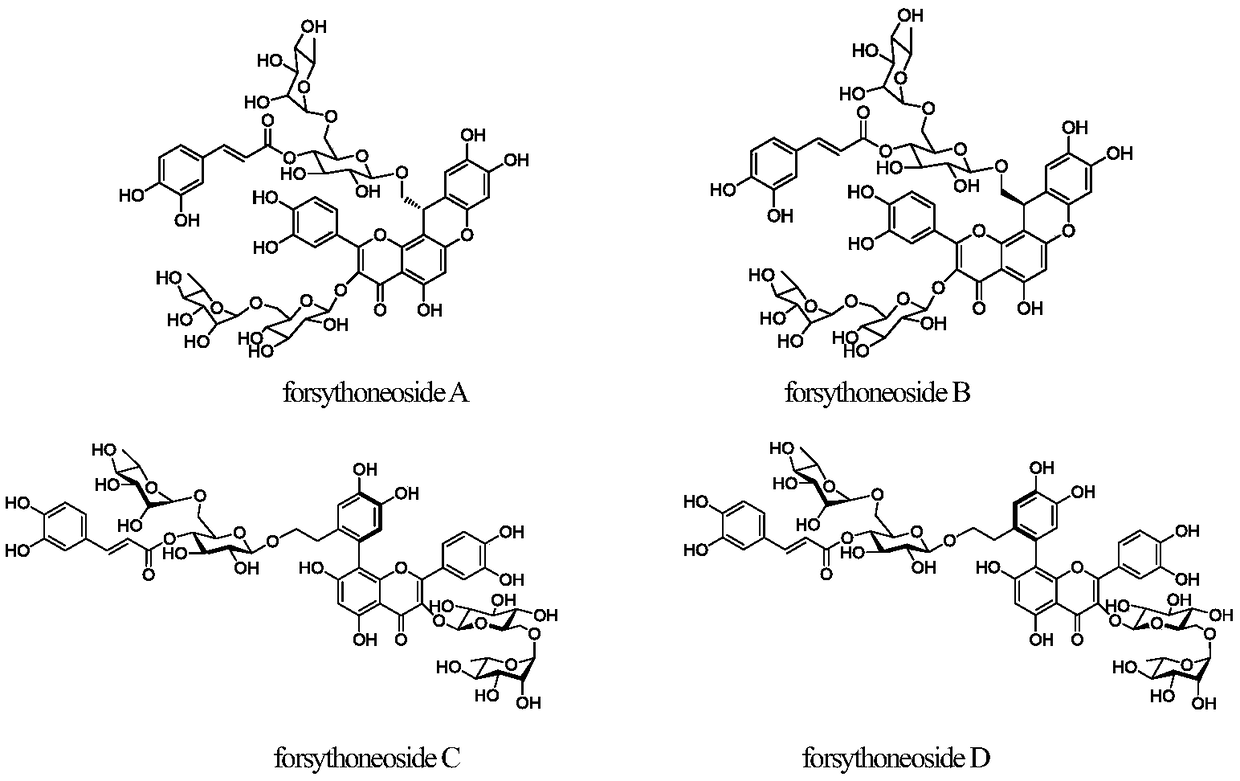
[0038] 10kg of forsythia medicinal material was reflux extracted with 20L of 70% ethanol solvent for 3 times, each time for 2 hours, the extract was concentrated to obtain 1.4kg of extract, the density of the extract was adjusted to 1.10 with distilled water, and 2L of petroleum ether was used in turn , chloroform, ethyl acetate and n-butanol were extracted 3 times successively, and the n-butanol part reclaimed the solvent to obtain 450 g of medicinal extract, which was dispersed and dissolved in distilled water (4L), and the supernatant was passed through a D101 type macroporous adsorption resin with a filling volume of 10L. Use water, 15% ethanol, 30% ethanol, 50% ethanol, 95% ethanol gradient (20L) for elution, collect the 50% ethanol elution fraction, recover the solvent and go through Sephadex LH-20 gel column chromatography, ODS reverse phase The above compounds forsythoneoside A (30 mg), forsythoneoside B (25 mg), forsythoneoside C (15 mg), and forsythoneoside D (15 mg) ...
experiment example 1
[0051] Effects of Forsythia Compounds on Nerve Cells (PC12) Injury in Vitro
[0052] Take the PC12 cells (simulated neuron cells) that cover a single layer, discard the original culture medium, add DMEM complete culture medium with 5% FBS and 5% horse serum, and blow gently with a pipette to completely disperse the cells. 4 Cells / mL density, 100 μL per well was inoculated in 96 culture plates treated with poly-lysine (0.1 mg / mL) in advance, cultured for 24 hours and then used for experiments. The experiment was divided into blank group, model group and drug-dosed group. The blank group was given complete medium, the rotenone model group was added with a complete medium with a final concentration of 4 μM rotenone to act on the cells for 48 hours (simulated PD model), and the oxygen-glucose deprivation (OGD) group was used with a low-glucose complete medium with a final concentration of 5 mM Sodium dithionite acts on cells for 24h (simulating cerebral ischemia model), Aβ 25-35...
experiment example 2
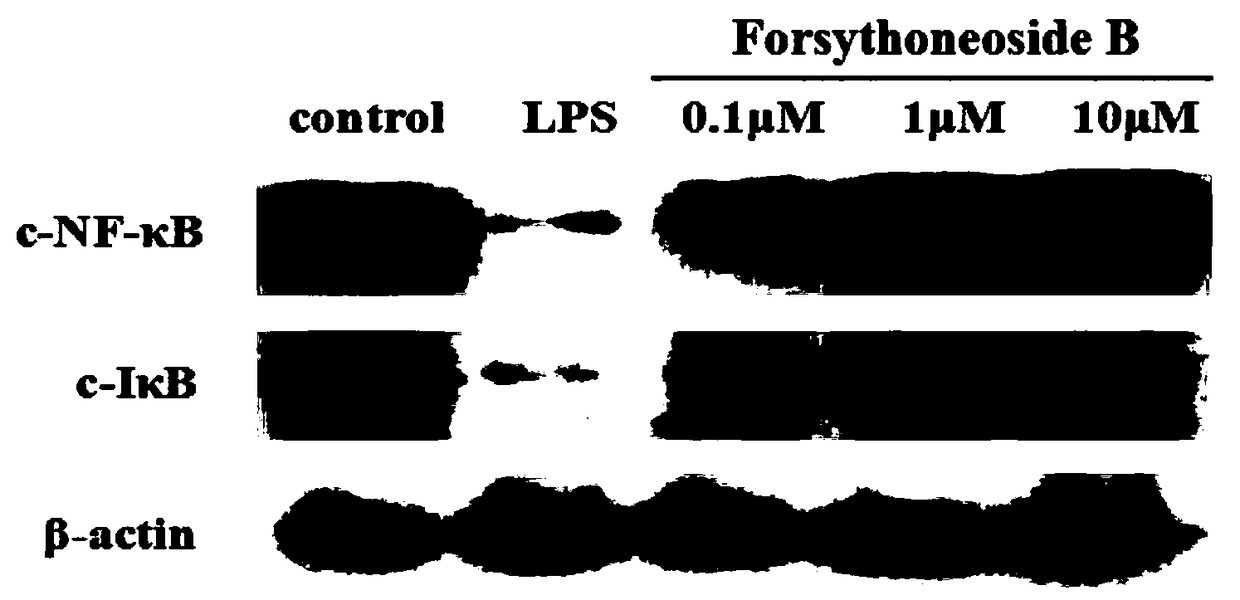
[0062] Effects of forsythia compounds on the activation of microglial cells (BV2) induced by lipopolysaccharide (LPS) in vitro
[0063] Take the BV2 cells (simulated neuroglial cells) that cover a single layer, discard the original culture medium, add 10% FBS DMEM complete culture medium, and blow gently with a pipette to completely disperse the cells. 5 Cells / mL density, 300 μL per well was inoculated in a 24-well culture plate treated with polylysine (0.1 mg / mL) in advance, and cultured for 24 hours before use in the experiment. The experiment was divided into blank group, model group and drug-dosed group. The blank group was given complete medium, and the LPS model group was given complete medium with a final concentration of 100ng / mL LPS (simulating neuroinflammation model). In the drug-dosing group, 10 μM, 1 μM or 0.1 μM of the test compound was added while modeling. The cells were treated for 24 hours, the cell morphology was observed under a microscope, the supernatan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com