Heptahydrate crystal and preparation method
A technology of zinc sulfate heptahydrate and crystals, which is applied in the field of waste water resource treatment, can solve problems such as complex production process, low product purity, and poor thermal stability, and achieve simple and easy-to-control operating conditions, smooth crystal surface, and good thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
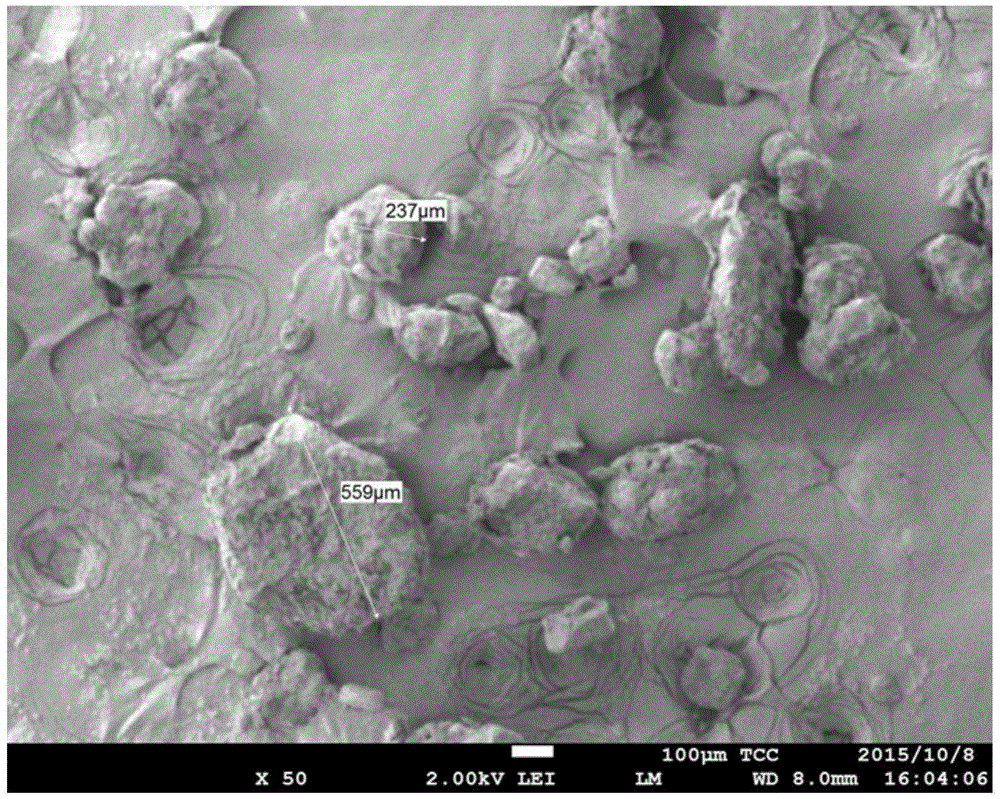
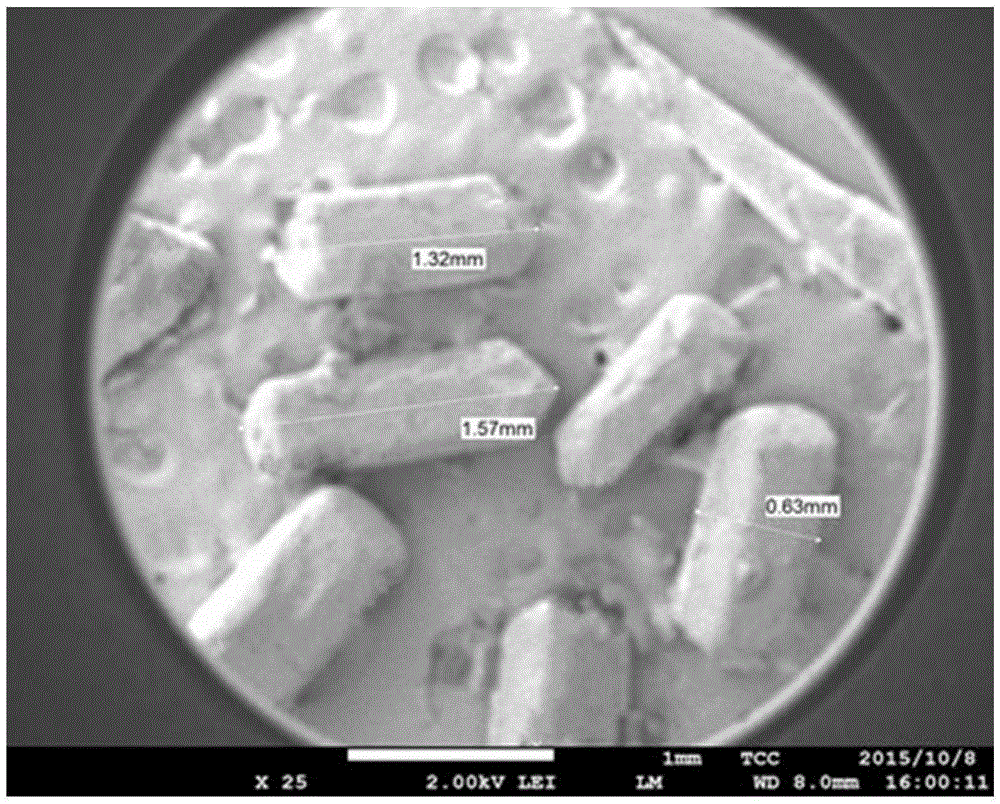
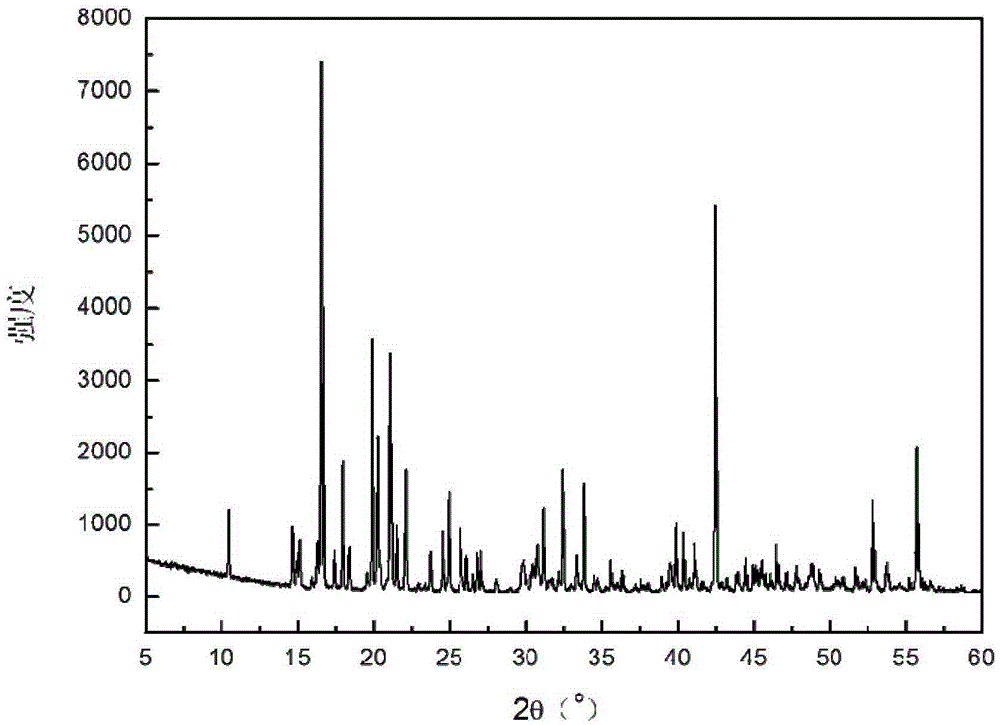
Image
Examples
Embodiment 1
[0033]Weigh 200.0 g of a binary solvent of methanol and ultrapure water, and the mass fraction of methanol is 20%. Add refined concentrated sulfuric acid dropwise, the mass ratio of sulfuric acid to binary solvent is 0.5:1; according to the molar ratio of zinc source and sulfuric acid as 1:1, add zinc carbonate three times, the reaction temperature is 50°C, and the reaction time is 30min; Add refined concentrated sulfuric acid, the molar ratio of zinc source to sulfuric acid is 1:0.25, and react for 30 minutes; lower the temperature of the solution to 5.0°C at a cooling rate of 0.2°C / min, and keep the temperature constant for 30 minutes; Pure water binary solvent (w methanol = 20%) is used as detergent to wash the filter cake, and the volume ratio of detergent to initial methanol and ultrapure water binary solvent (200.0g) is 0.5:1; The obtained filter cake is dried under 0.08MPa to obtain 264.74g of prismatic zinc sulfate heptahydrate crystals, the purity is 99.8%, and the yi...
Embodiment 2
[0035] Weigh 200.0 g of a binary solvent of ethanol and ultrapure water, and the mass fraction of ethanol is 50%. Add refined concentrated sulfuric acid dropwise, the mass ratio of sulfuric acid to binary solvent is 0.2:1; according to the molar ratio of zinc source and sulfuric acid as 1:1, add zinc oxide three times, the reaction temperature is 60°C, and the reaction time is 10 minutes; Add refined concentrated sulfuric acid, the molar ratio of zinc source and sulfuric acid is 1:0.05, and react for 30 minutes; lower the temperature of the solution to 10.0°C at a cooling rate of 0.5°C / min, and keep the temperature constant for 60 minutes; suction filter the suspension, and use ethanol and super Pure water binary solvent (w ethanol=50%) is used as detergent to wash the filter cake, and the volume ratio of detergent to initial methanol and ultrapure water binary solvent (200.0g) is 0.5:1; The obtained filter cake was dried under 0.10MPa to obtain 106.58g of prismatic zinc sulfa...
Embodiment 3
[0037] Weigh 200.0 g of a binary solvent of isopropanol and ultrapure water, and the mass fraction of isopropanol is 35%. Add refined concentrated sulfuric acid dropwise, the mass ratio of sulfuric acid to binary solvent is 0.3:1; add the binary mixture of zinc oxide and zinc hydroxide three times according to the molar ratio of zinc source to sulfuric acid as 1:1 (molar ratio is 1: 1), the reaction temperature is 55°C, and the reaction time is 20min; add refined concentrated sulfuric acid for the second time, the molar ratio of zinc source to sulfuric acid is 1:0.10, and react for 20min; the solution is reduced to 5.0°C at a cooling rate of 1.0°C / min ℃, constant temperature for 60min; the suspension was filtered with suction, and the filter cake was washed with a binary solvent of isopropanol and ultrapure water (w isopropanol=35%), and the detergent was mixed with the initial methanol and ultrapure water. The volume ratio of the primary solvent (200.0g) was 0.5:1; the result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com