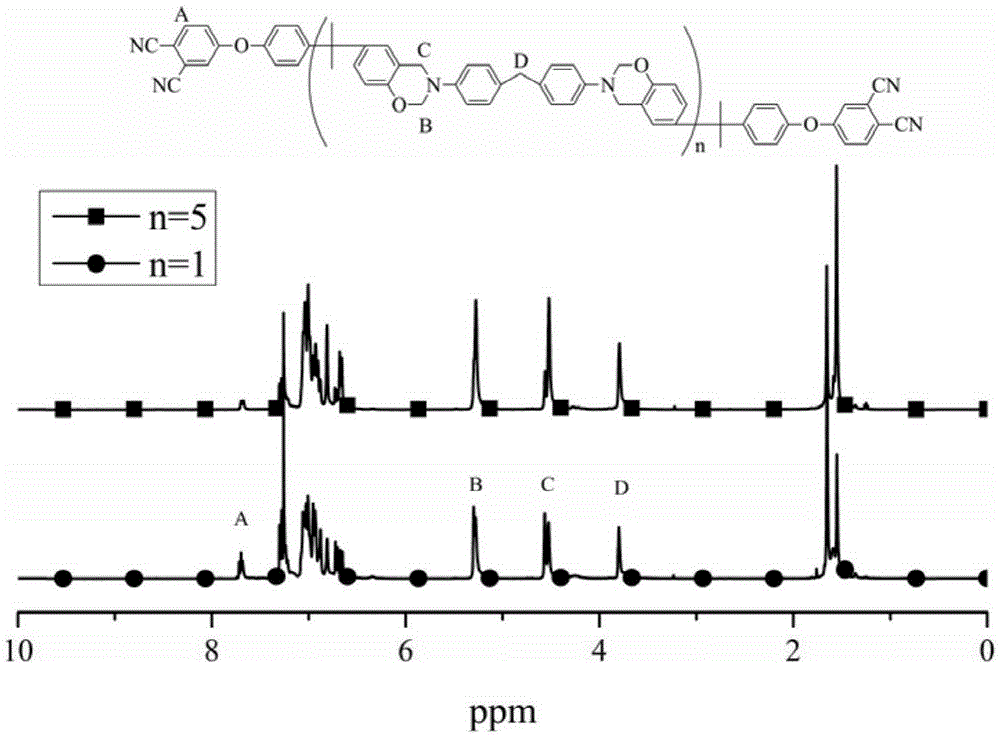
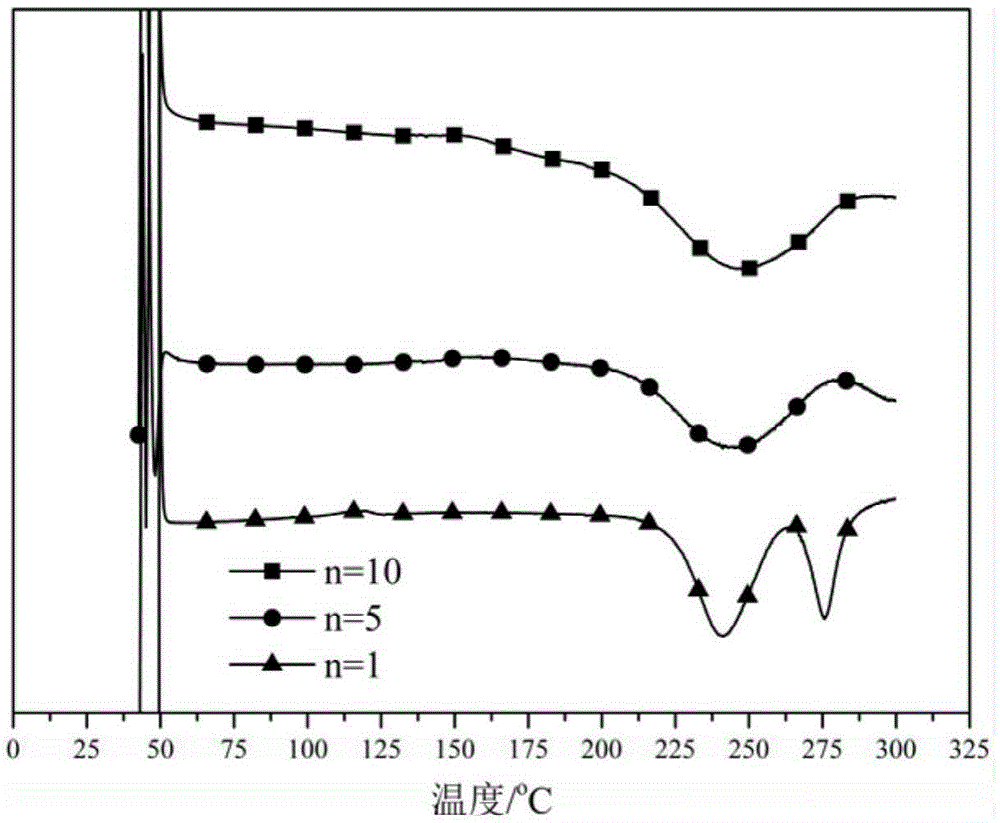
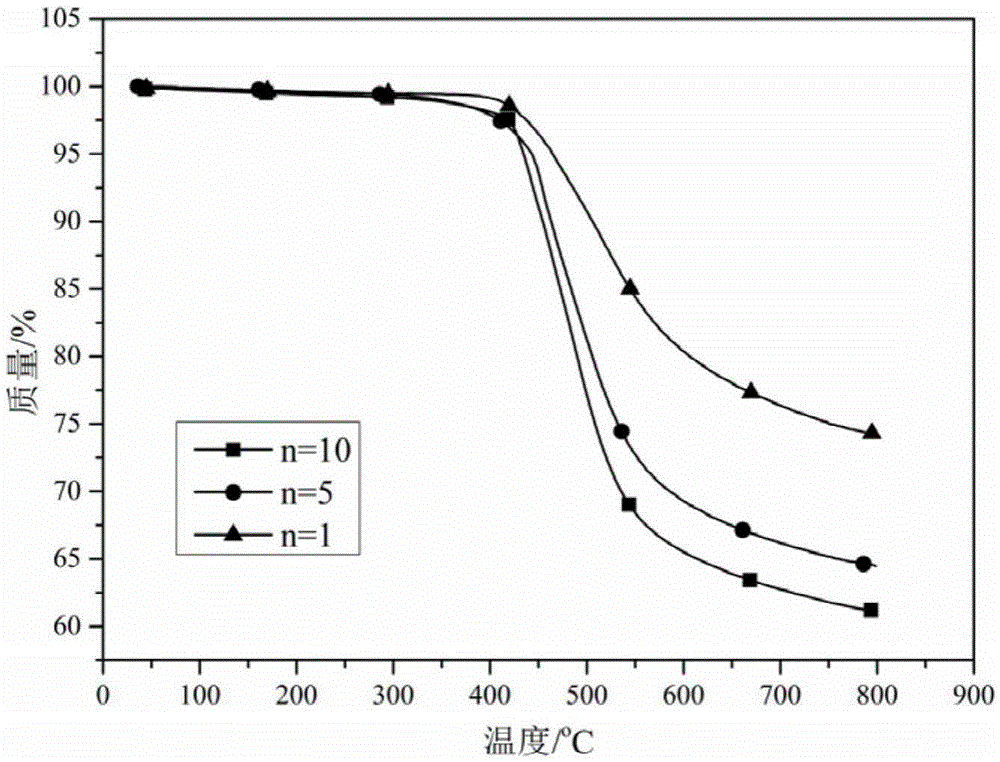
Polybenzoxazine connected bisphthalonitrile monomer as well as preparation method and application thereof
A bis-phthalonitrile and polybenzoxazine technology, applied in the field of bis-phthalonitrile monomer and its preparation, can solve the problems of high curing reaction temperature, high brittleness of cured products, insufficient thermal stability, etc. , to achieve the effect of low water absorption and high carbon residue rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 (n=10)
[0042] (1) Add 7.93g (0.04mol) 4,4'-diaminodiphenylmethane, 4.80g (0.16mol) paraformaldehyde and 150mL dioxane to a 250mL flask equipped with a magnetic stirrer; the mixture After stirring at room temperature for 30 minutes, 10.04 g (0.044 mol) of bisphenol A was added, and then the temperature was gradually raised to 100°C for reflux and maintained for 8 hours; after the reaction was lowered to room temperature, anhydrous sodium sulfate was added, dried for 12 hours and filtered, and the filtrate was passed through The solvent was removed by rotary evaporation to obtain compound 1;
[0043] (2) Dissolve the compound 1 prepared in step (1) with 150 mL of dry DMF, and add 1.39 g (0.008 mol) 4-nitrophthalonitrile and 2.21 g (0.016 mol) After adding anhydrous potassium carbonate, stir and react at 60°C for 2 hours. After the reaction was completed, the reaction mixture was poured into a large amount of water to precipitate, and the precipitate was coll...
Embodiment 2
[0044] Example 2 (n=5)
[0045] (1) Add 7.93g (0.04mol) 4,4'-diaminodiphenylmethane, 4.80g (0.16mol) paraformaldehyde and 150mL chloroform in a 250mL flask equipped with a magnetic stirrer; After stirring at room temperature for 30 minutes, 10.96 g (0.048 mol) of bisphenol A was added, and then the temperature was gradually raised to 60° C. for reflux and kept for 12 hours. After the reaction was lowered to room temperature, anhydrous sodium sulfate was added, dried for 12 hours and then filtered, and the filtrate was removed by rotary evaporation to obtain compound 1;
[0046] (2) Dissolve the compound 1 obtained in step (1) with 150 mL of dry DMF, and add 2.77 g (0.016 mol) 4-nitrophthalonitrile and 4.42 g (0.032 mol) After the addition of anhydrous potassium carbonate, stir the reaction at room temperature (25°C) for 24 hours. After the reaction was completed, the reaction mixture was poured into a large amount of water to precipitate, and the precipitate was collected by...
Embodiment 3
[0047] Embodiment 3 (n=1)
[0048](1) In a 250mL flask equipped with a magnetic stirrer, add 7.93g (0.04mol) 4,4'-diaminodiphenylmethane, 4.80g (0.16mol) paraformaldehyde and 150mL chloroform; After stirring at room temperature for 30 minutes, 18.26 g (0.08 mol) of bisphenol A was added, and then the temperature was gradually raised to 60° C. for reflux and kept for 12 hours. After the reaction was lowered to room temperature, anhydrous sodium sulfate was added, dried for 12 hours and then filtered, and the filtrate was removed by rotary evaporation to obtain compound 1;
[0049] (2) Dissolve the compound 1 prepared in step (1) with 150 mL of dry DMF, and add 13.85 g (0.08 mol) 4-nitrophthalonitrile and 22.11 g (0.16 mol) After the addition of anhydrous potassium carbonate, stir the reaction at room temperature (25°C) for 24 hours. After the reaction was completed, the reaction mixture was poured into a large amount of water to precipitate, and the precipitate was collected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com