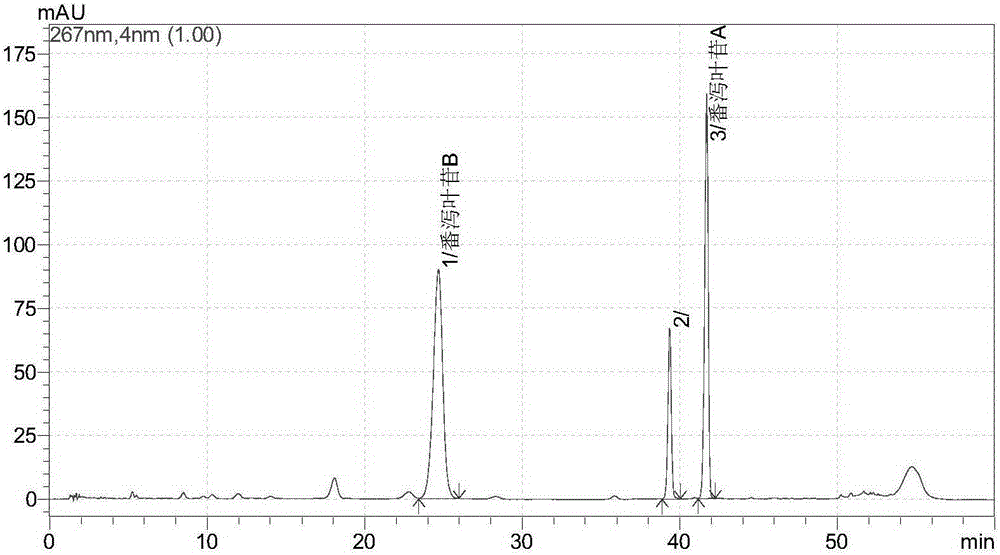
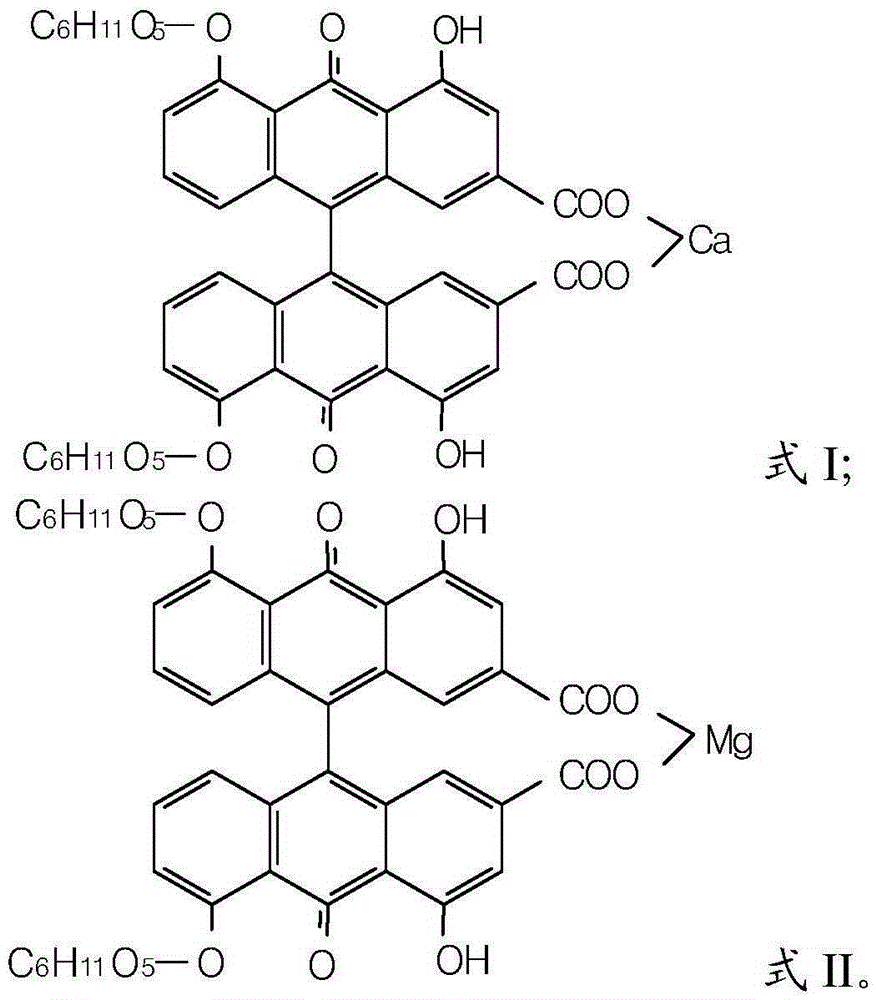
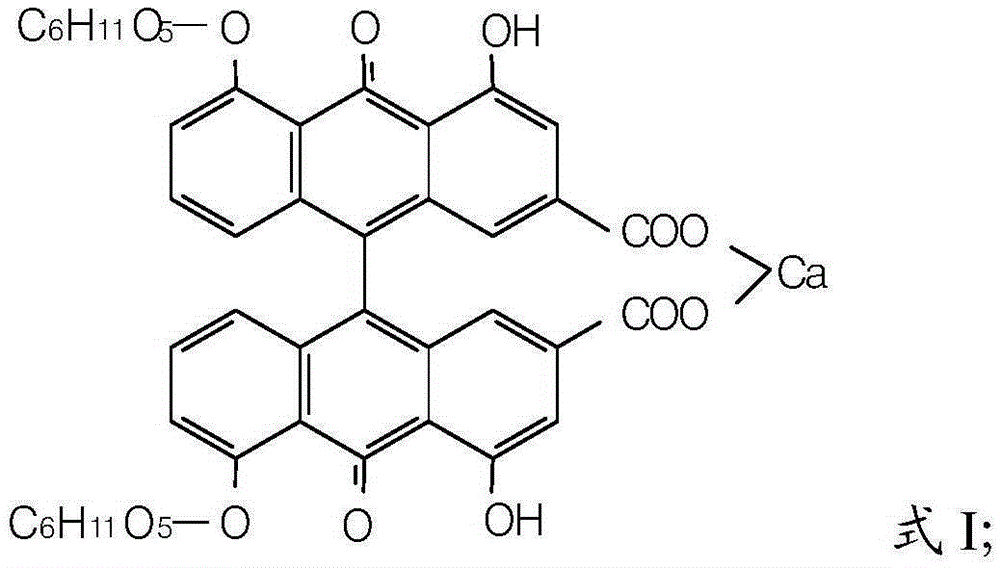
Sennoside A.B salt compound as well as preparation method and application thereof
A technology of sennosides and compounds, which is applied in the field of medicine, can solve problems such as unfavorable popularization and clinical application of patent medicines for treating constipation, poor stability of sennoside A·B, etc., to achieve favorable promotion and clinical application, stable active ingredients, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Specifically, the preparation method of the sennoside A·B salt compound comprises the following steps:
[0035] (a) extracting senna leaves with an aqueous solution of sodium bicarbonate to obtain an extract, and filtering the extract through a 100-mesh to 200-mesh sieve to obtain a filtered extract;
[0036] (b) After the filtered extract obtained in step (a) is acidified, the acidified product obtained is subjected to solid-liquid separation to obtain liquid phase component A, and the liquid phase component A is filtered through molecular interception Obtain fine filtrate;
[0037] (c) mixing activated clay with the fine filtrate obtained in step (b), stirring and adsorbing, separating the adsorbed mixture from solid to liquid, and eluting the obtained solids successively with water and water-containing alcohol, and the eluted The solid is subjected to solid-liquid separation to obtain the liquid phase component B;
[0038] (d) concentrating the liquid phase compone...
Embodiment 1
[0064] Crush the senna leaves of the prescribed amount, add 10 times the mass of 0.5wt% sodium bicarbonate aqueous solution to carry out stirring and extraction at room temperature for 3 hours, collect the extract, filter, add 0.5mol / L hydrochloric acid to the filtrate to adjust the pH value to 4, and stir , stand still, filter to obtain the medicinal solution, carry out the molecular interception filtration of the obtained medicinal solution through a ceramic membrane filter, the ceramic membrane filter aperture is 0.2 μm, and obtain the fine filtrate; add 5% activated clay to the fine filtrate, stir for 3 hours, Filter, add 5 times the volume of activated clay to wash with water to remove impurities, then elute with 90wt% ethanol solution, collect the eluate, add anhydrous calcium sulfate to remove water, and then remove the impurities at a temperature of 60°C and a vacuum of -0.08MPa Concentrate under reduced pressure for 2 hours;
[0065] Mix the obtained concentrate with ...
Embodiment 2
[0069] Get the senna leaf of recipe quantity, pulverize, add 0.2wt% sodium bicarbonate aqueous liquid of 8 times amount and carry out room temperature stirring extraction for 2 hours, collect extract, filter, add the hydrochloric acid of 0.5mol / L to filtrate, adjust the pH value to 3. Stir, stand still, and filter to obtain the filtrate, carry out molecular interception filtration through the obtained medicinal liquid through a ceramic membrane filter, the aperture of the ceramic membrane filter is 0.1 μm, and obtain the fine filtrate; 5% activated clay of the fine filtrate is stirred for 2 hour, filter, and the gained activated clay is washed with water of 3 times the volume to remove impurities, then eluted with 90wt% ethanol solution, collects the eluent, adds anhydrous calcium sulfate to remove moisture, and at a temperature of 80°C and a vacuum of - Concentrate under reduced pressure for 1 hour under the condition of 0.1MPa;
[0070] Mix the obtained concentrate with 0.5m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com