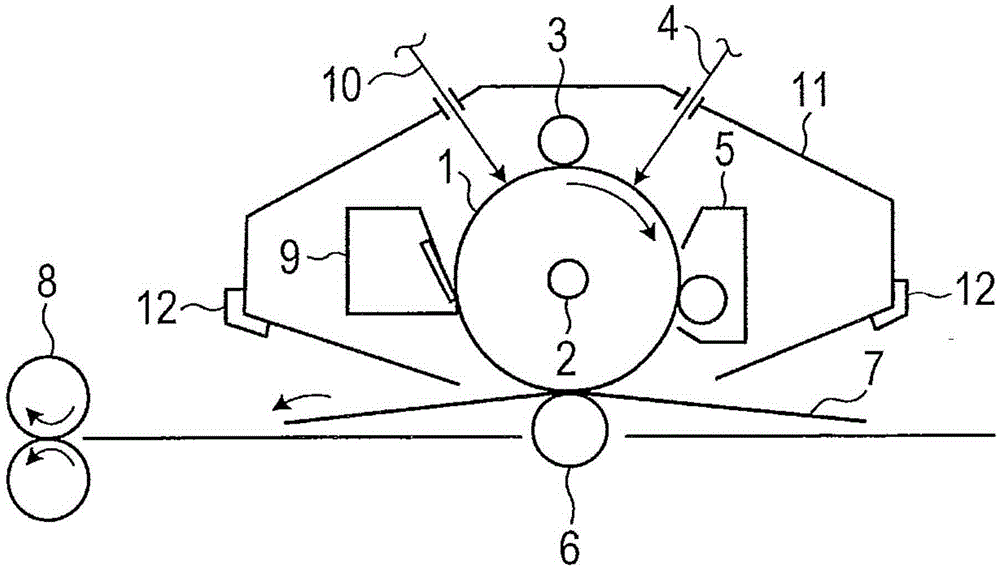
Electrophotographic photosensitive member, process cartridge, electrophotographic apparatus and phthalocyanine crystal
A technology of electrophotography and photosensitive components, which is applied in the fields of optics, electrical recording, chemical instruments and methods, etc., can solve problems such as conversion difficulties and achieve excellent performance effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
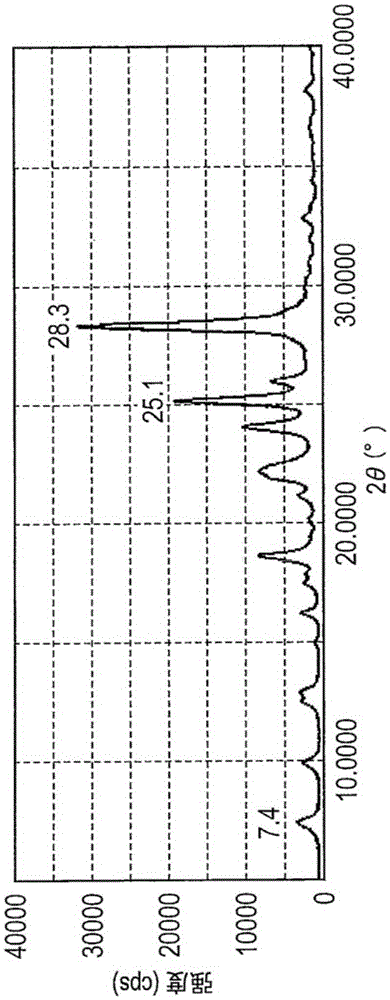
[0131] Hydroxygallium phthalocyanine was produced in the same manner as in (Synthesis Example 1) and (Example 1-1) described in Japanese Patent Application Laid-Open No. 2011-94101 as described below. Under an atmosphere of nitrogen flow, 5.46 parts of phthalonitrile and 45 parts of α-chloronaphthalene were put into the reaction kettle, and then heated to a temperature of 30° C., and kept at this temperature. Next, 3.75 parts of gallium trichloride were injected thereinto at the temperature (30° C.). When charged, the mixed liquor had a water content of 150 ppm. The temperature was then raised to 200°C. The reaction was carried out at a temperature of 200° C. for 4.5 hours under a nitrogen flow atmosphere, and then cooled to a temperature of 150° C., and the product was filtered. The obtained filtrate was dispersed and washed with N,N-dimethylformamide at a temperature of 140° C. for 2 hours, and then filtered. The obtained filtrate was washed with methanol and dried to obt...
Embodiment 1-2
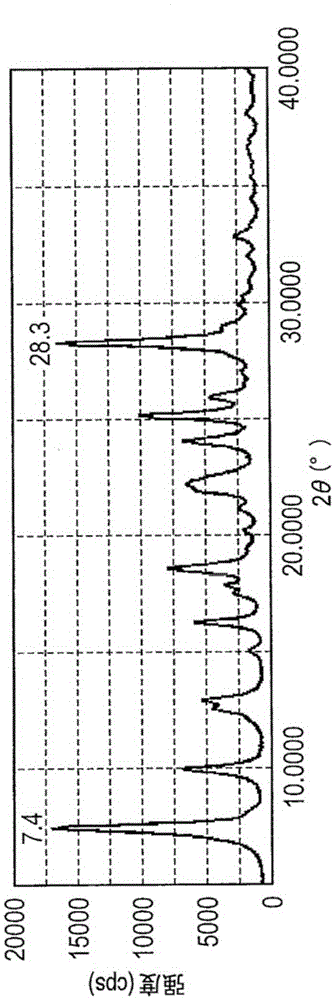
[0135] Except that in Example 1-1, 0.5 parts of exemplary compound (1) was replaced with 1.0 part of exemplary compound (1), and the milling time was changed from 70 hours to 50 hours, by the same method as in Example 1-1 The treatment obtained 0.44 parts of hydroxygallium phthalocyanine crystals. The powder X-ray diffraction pattern of the obtained crystals is in image 3 shown in .
[0136] By NMR measurement, it was confirmed that 0.67% by mass of Exemplary Compound (1) and 2.14% by mass of N,N-dimethylformamide were contained in the crystal.
Embodiment 1-3
[0138] Except that in Example 1-1, 9.5 parts of N,N-dimethylformamide was replaced with 9.5 parts of dimethyl sulfoxide, and the grinding time was changed from 70 hours to 50 hours, by the same method as in Example 1- 1 The same treatment obtained 0.41 parts of hydroxygallium phthalocyanine crystals. The powder X-ray diffraction patterns of the obtained crystals are image 3 same.
[0139] By NMR measurement, it was confirmed that 0.79% by mass of Exemplary Compound (1) and 2.20% by mass of dimethyl sulfoxide were contained in the crystal. Since Exemplary Compound (1) is liquid and compatible with dimethyl sulfoxide, it was found that Exemplary Compound (1) was contained within the phthalocyanine crystal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com