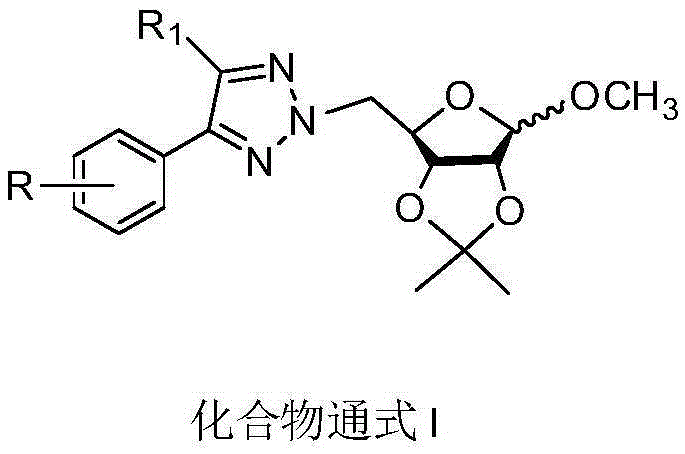
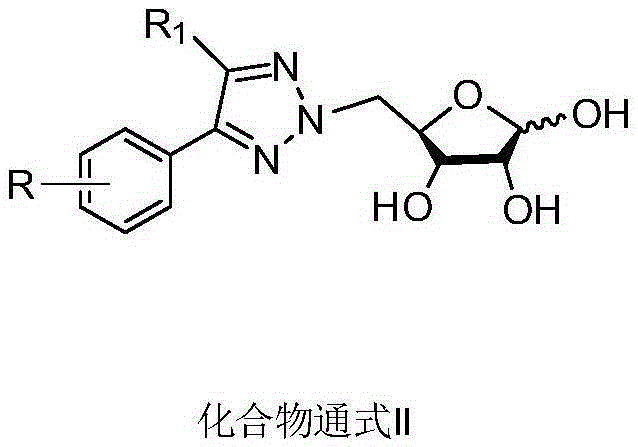
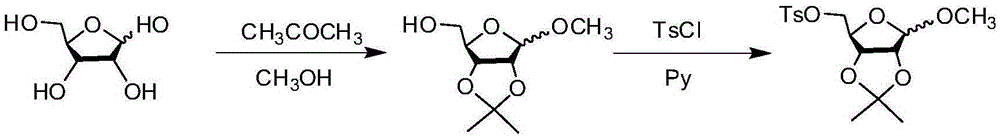
N2-glycosyl-substituted 1,2,3-triazole compound and synthesis method and application thereof
A triazole compound and compound technology, applied in N2-glycosyl-substituted 1,2,3-triazole compounds and their synthesis and application fields, achieving the effects of high yield, strong inhibitory ability, and easy control of synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] Step 1: Compound a 1 Synthesis,
[0051] Weigh and dissolve sodium azide (8.7g, 0.13mol) in 35mL of DMSO, heat and stir, dissolve phenylnitroalkene compound (16.63g, 0.11mol) in 30mL of DMSO, and slowly drop into the stack with a constant pressure dropping funnel. In the sodium nitride solution, one drop is added dropwise at a rate of 3 seconds, and the reaction temperature is 80°C. After the dropwise addition is complete, stop heating and continue stirring, and cool to room temperature; add ammonium chloride solution to the mixed solution, extract with ethyl acetate, extract the organic phase, wash the organic layer with saturated brine again, and then wash with anhydrous sulfuric acid The organic layer was dried over sodium, filtered, and concentrated under reduced pressure to obtain a yellow-brown solid. Finally, the obtained product was washed with dichloromethane in a small amount and several times to obtain about 14.51 g of a white solid with a yield...
Embodiment 2~5
[0058] The reaction steps of embodiment 2~5 refer to step 1, step 2 and step 3 of embodiment 1, participate in the reaction compound list as follows:
[0059]
[0060] c 2 : 1 HNMR (600MHz, CDCl 3 )δ7.76–7.62(m,2H),7.57–7.51(m,1H),7.41(t,J=7.8Hz,1H),7.32(dd,J=14.4,7.3Hz,1H),5.01(s ,1H),4.85(dd,J=11.4,5.9Hz,1H),4.78–4.73(m,1H),4.69(dd,J=6.0,2.0Hz,1H),4.54–4.47(m,2H), 3.38(d, J=3.6Hz, 3H), 2.45(d, J=16.2Hz, 3H), 1.44(d, J=11.4Hz, 3H), 1.29(s, 3H).
[0061] c 3 : 1 HNMR (400MHz, CDCl 3 )δ7.68(d, J=7.8Hz, 1H), 7.37(t, J=4.2Hz, 2H), 7.27(s, 1H), 5.03(s, 1H), 4.90(d, J=6.0Hz, 1H), 4.76(d, J=7.2Hz, 1H), 4.70(d, J=6.0Hz, 1H), 4.55(dd, J=7.2, 3.0Hz, 2H), 3.39(s, 3H), 2.27( s,3H),1.48(s,3H),1.31(s,3H).
[0062] c 4 : 1 HNMR (600MHz, CDCl 3 )δ8.15(s,1H),7.83(d,J=8.4Hz,1H),7.48(s,1H),7.30(d,J=8.4Hz,1H),5.03(s,1H),4.87( d,J=6.0Hz,1H),4.79(t,J=7.2Hz,1H),4.71(d,J=6.0Hz,1H),4.62(dd,J=12.0,7.5Hz,2H),3.41( s,3H),1.47(s,3H),1.31(s,3H).
[0063] c 5 : 1 HNMR (600MHz, CDCl 3 )...
Embodiment 6
[0065]
[0066] Step 1, Step 2 and Step 3 of Embodiment 6 refer to Embodiment 1.
[0067] Step 4:
[0068] the white solid c 6 Add dilute sulfuric acid solution, stir at 80°C, react for 2 days, monitor the reaction by TLC, stop heating after the reaction is over, cool to room temperature, extract with ethyl acetate, extract the organic phase, wash the organic layer with saturated brine again, and then The organic layer was dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain a light yellow solid, and ethyl acetate / petroleum ether (1:1) column chromatography obtained a white solid. The white solid is compound d 6 .
[0069] 1 HNMR(600MHz,DMSO)δ8.14(s,1H),7.76(d,J=8.4Hz,2H),7.01(d,J=8.4Hz,2H),5.06(s,1H),4.94(d, J=6.0Hz, 1H), 4.62(dd, J=13.8, 3.2Hz, 1H), 4.47(dd, J=13.8, 8.7Hz, 1H), 4.22(d, J=6.0Hz, 1H), 4.08– 4.00(m,1H),3.41(d,J=10.2Hz,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com