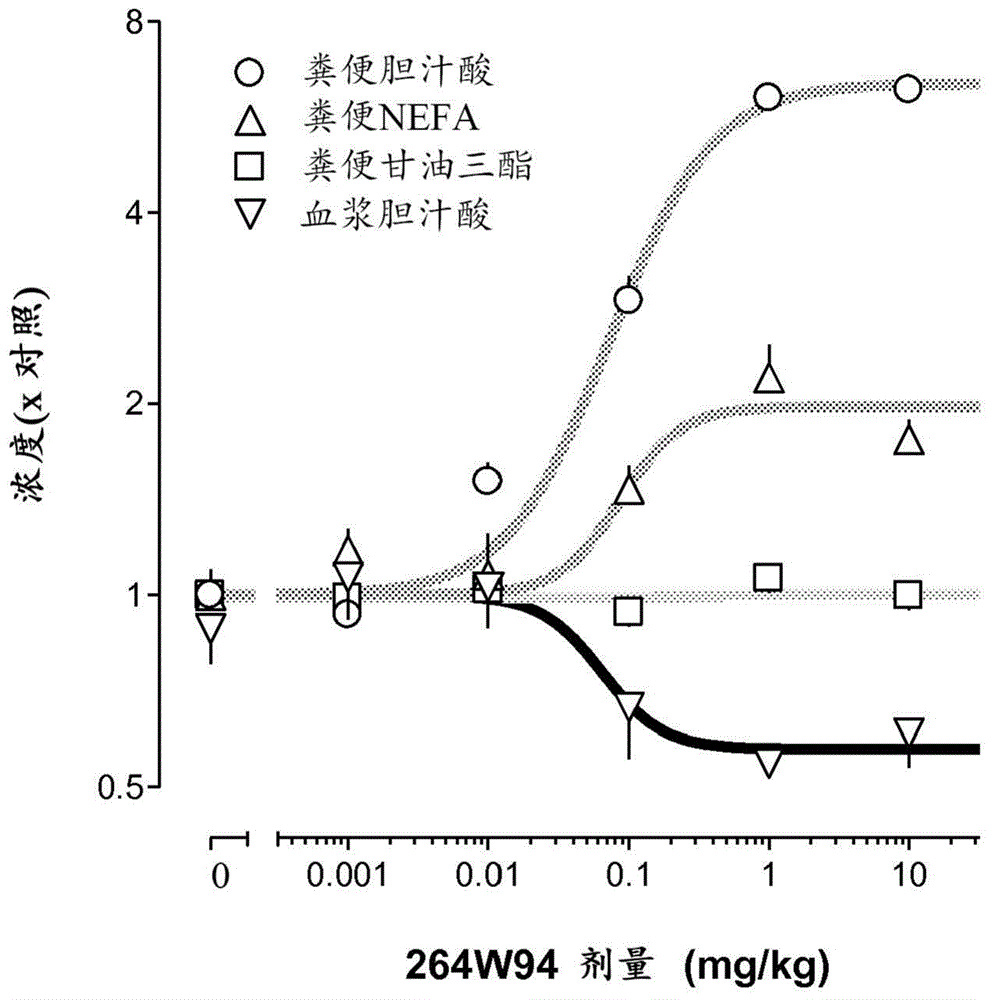
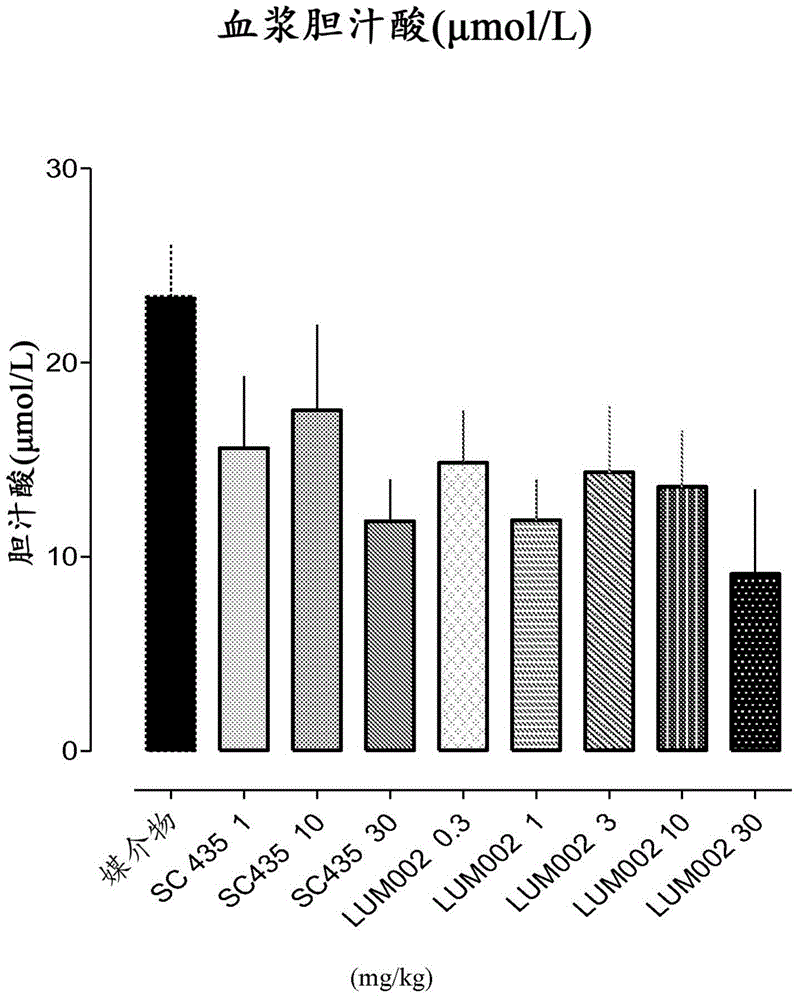
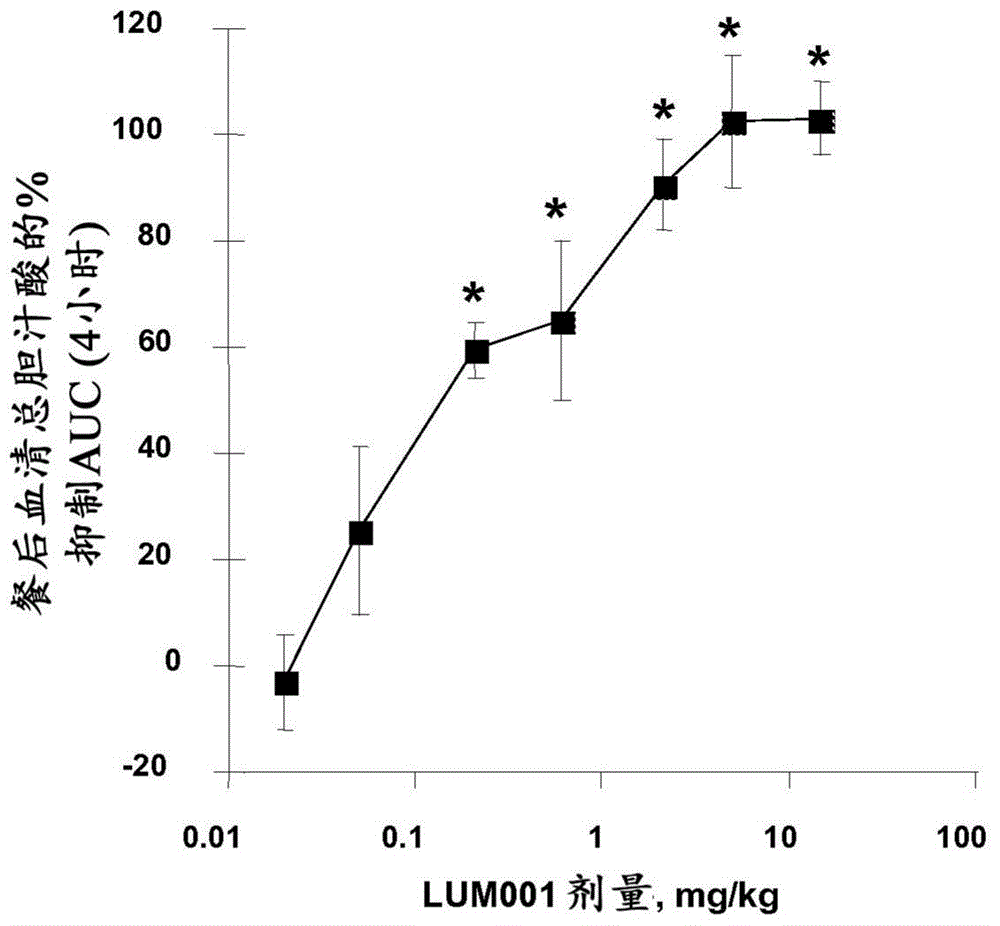
Bile acid recycling inhibitors for treatment of barrett's esophagus and gastroesophageal reflux disease
A technique for gastroesophageal reflux and bile acids, applied to treat or improve Barrett's esophagus, pharmaceutical composition for treating GERD, pharmaceutical composition for treating or improving Barrett's esophagus and GERD, treating Barrett's esophagus and GERD A pharmaceutical composition, a pharmaceutical composition for the treatment of Barrett's esophagus, for the treatment or amelioration of the GERD field, capable of addressing the increased risk etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0766] Example 1: Synthesis of 1-phenethyl-1-((1,4-diazabicyclo[2.2.2]octyl)pentyl)iminodiiminoformamide iodide salt
[0767]
[0768] step 1: Synthesis of 5-(1,4-diazabicyclo[2.2.2]octyl)-1-iodopentane iodide salt
[0769]
[0770] 1,4-Diazabicyclo[2.2.2]octane was suspended in THF. Diiodopentane was added dropwise, and the mixture was refluxed overnight. The reaction mixture was filtered.
[0771] Step 2: Synthesis of N-phenethyl-5-(1,4-diazabicyclo[2.2.2]octyl)-1-iodopentane iodide salt.
[0772]
[0773] 5-(1,4-Diazabicyclo[2.2.2]octyl)-1-iodopentane iodide salt was suspended in acetonitrile. Phenylethylamine was added dropwise, and the mixture was refluxed overnight. The reaction mixture was filtered.
[0774] Step 3: Synthesis of 1-phenethyl-1-((1,4-diazabicyclo[2.2.2]octyl)pentyl)iminodiiminoformamide iodide salt.
[0775] N-phenethyl-5-(1,4-diazabicyclo[2.2.2]octyl)-1-iodopentane iodide was heated with dicyanodiamide in n-butanol for 4 h . The ...
Embodiment 2
[0779] Example 2: In Vitro Assay for ASBT-Mediated Inhibition of Bile Acid Uptake
[0780]Baby hamster kidney (BHK) cells were transfected with cDNA of human ASBT. Cells were seeded in 96-well tissue culture plates at 60,000 cells / well. Assays were performed within 24 hours of inoculation.
[0781] On the day of the assay, wash the cell monolayer with 100 mL of assay buffer. The test compound was mixed with 6mM [ 14 C] Taurocholate was added together to each well (final concentration in each well was 3 mM [ 14 C] Taurocholate). Cell cultures were incubated at 37°C for 2h. Wells were washed with PBS. Scintillation fluid was added to each well and the cells were shaken for 30 minutes before measuring the amount of radioactivity in each well. Test compounds with significant ASBT inhibitory activity provide an assay in which low levels of radioactivity are observed in cells.
Embodiment 3
[0782] Example 3: In vitro assay for secretion of GLP-2
[0783] Human NCI-H716 cells were used as a model for L-cells. Two days before each assay experiment, cells were plated in Coated 12-well culture plates to induce cell adhesion. On the day of the assay, cells were washed with buffer. Cells were incubated with medium alone or with test compounds for 2 hours. Extracellular media were analyzed for the presence of GLP-2. Peptides in the medium were collected by reverse phase adsorption and the extracts were stored until assayed. ELISA was used to analyze the presence of GLP-2. Detection of increased GLP-2 levels in wells containing the test compound identifies the test compound as a compound that enhances GLP-2 secretion from L-cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com