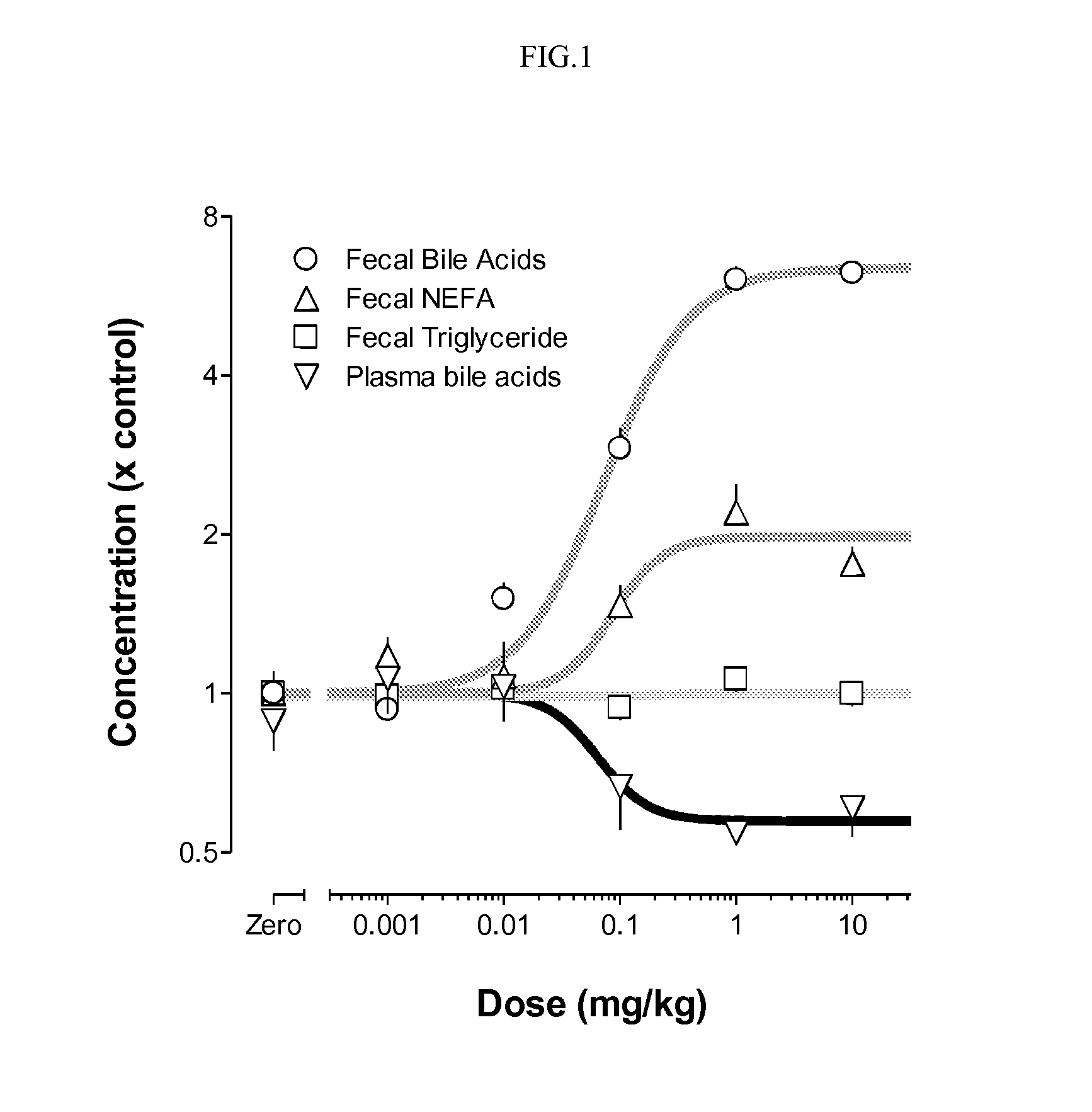
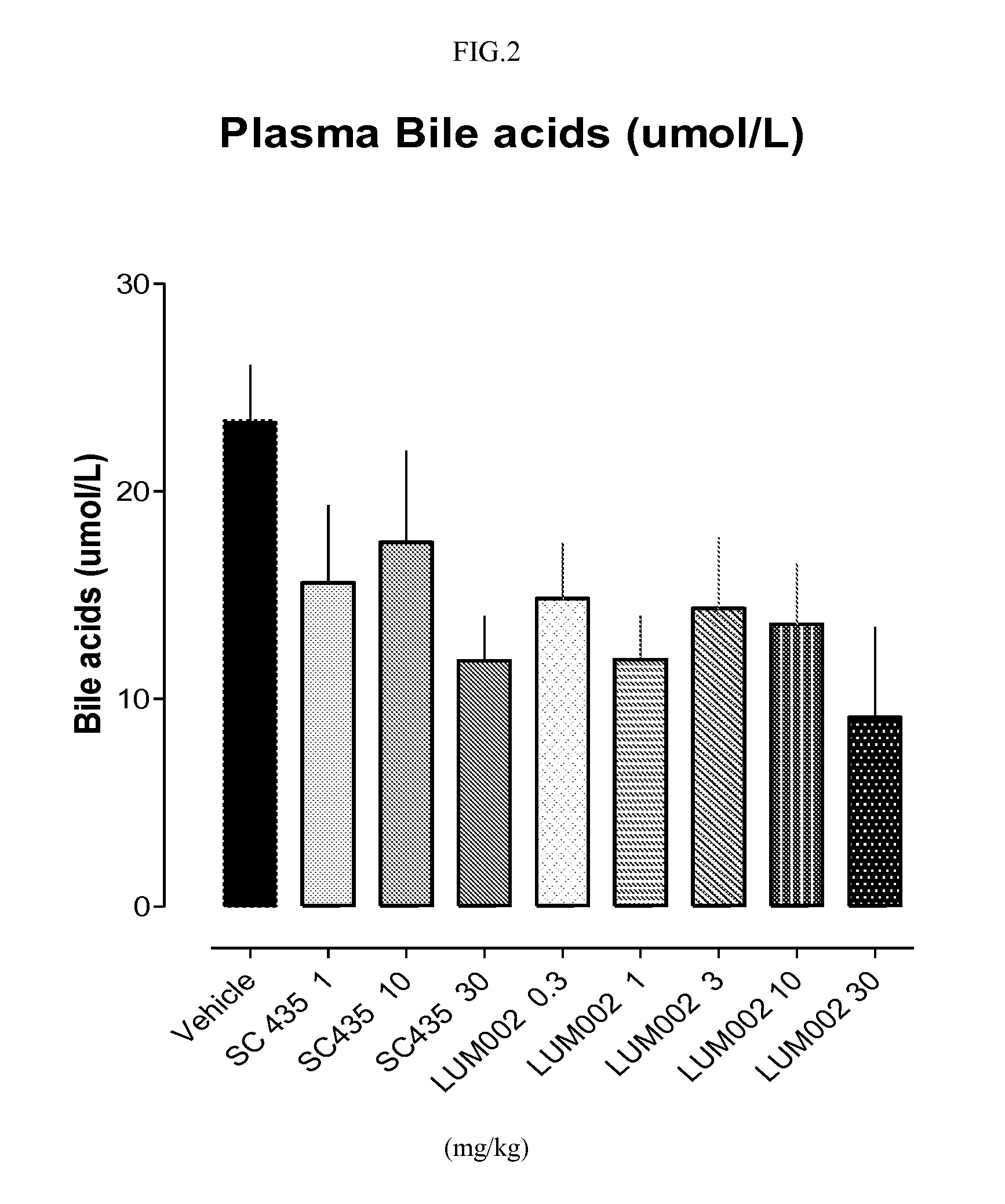
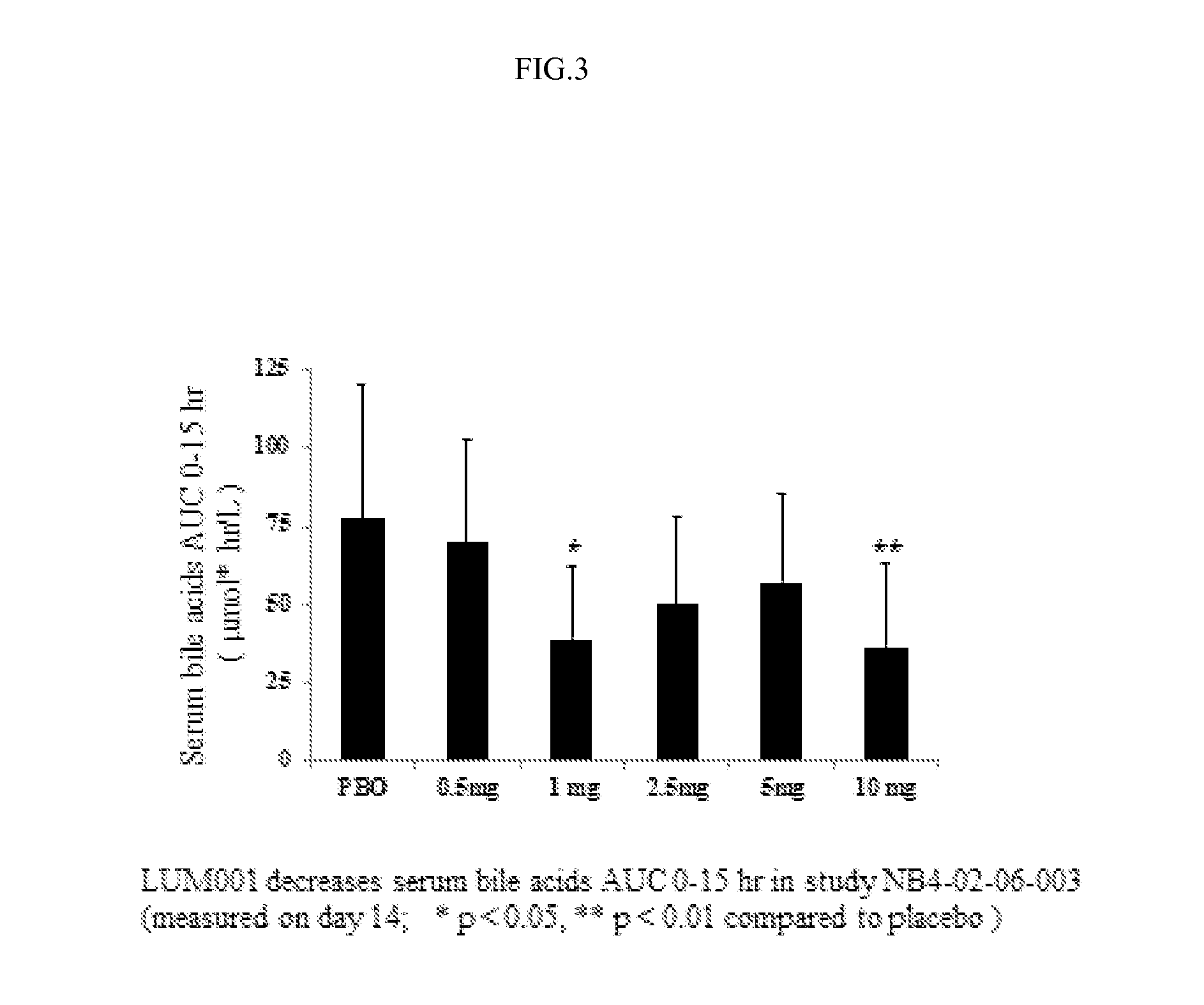
Bile acid recycling inhibitors for treatment of hypercholemia and cholestatic liver disease
a bile acid recycling inhibitor and liver disease technology, applied in the direction of esterified saccharide compounds, drug compositions, cyclic peptide ingredients, etc., can solve the problems of limited active treatment and prevention, reduce the recurrence of hypercholemia, and reduce the severity of symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 1-phenethyl-1-((1,4-diazabicyclo[2.2.2]octanyl)pentyl)imidodicarbonimidic diamide, iodide salt
[0654]
Step 1: Synthesis of 5-(1,4-diazabicyclo[2.2.2]octanyl)-1-iodo pentane, iodide salt
[0655]
[0656]1,4-diazabicyclo[2.2.2]octane is suspended in THF. Diiodopentane is added dropwise and the mixture is refluxed overnight. The reaction mixture is filtered.
Step 2: Synthesis of N-phenethyl-5-(1,4-diazabicyclo[2.2.2]octanyl)-1-iodo pentane, iodide salt
[0657]
[0658]5-(1,4-diazabicyclo[2.2.2]octanyl)-1-iodo pentane, iodide salt is suspended in acetonitrile. Phenethylamine is added dropwise and the mixture is refluxed overnight. The reaction mixture is filtered.
Step 3: Synthesis of 1-phenethyl-1-((1,4-diazabicyclo[2.2.2]octanyl)pentyl)imidodicarbonimidic diamide, iodide salt
[0659]N-phenethyl-5-(1,4-diazabicyclo[2.2.2]octanyl)-1-iodo pentane, iodide salt is heated with dicyanodiamide in n-butanol for 4 h. The reaction mixture is concentrated under reduced pressure.
[0660]The compounds i...
example 2
In Vitro Assay for Inhibition of ASBT-Mediated Bile Acid Uptake
[0661]Baby hamster kidney (BHK) cells are transfected with cDNA of human ASBT. The cells are seeded in 96-well tissue culture plates at 60,000 cells / well. Assays are run within 24 hours of seeding.
[0662]On the day of the assay the cell monolayer is washed with 100 mL of assay buffer. The test compound is added to each well along with 6 mM [14C] taurocholate in assay buffer (final concentration of 3 mM [14C] taurocholate in each well). The cell cultures are incubated for 2 h at 37° C. The wells are washed with PBS. Scintillation counting fluid is added to each well, the cells are shaken for 30 minutes prior to measuring amount of radioactivity in each well. A test compound that has significant ASBT inhibitory activity provides an assay wherein low levels of radioactivity are observed in the cells.
example 3
In Vitro Assay for Secretion of GLP-2
[0663]Human NCI-H716 cells are used as a model for L-cells. Two days before each assay experiment, cells are seeded in 12-well culture plates coated with Matrigel® to induce cell adhesion. On the day of the assay, cells are washed with buffer. The cells are incubated for 2 hours with medium alone, or with test compound. The extracellular medium is assayed for the presence of GLP-2. Peptides in the medium are collected by reverse phase adsorption and the extracts are stored until assay. The presence of GLP-2 is assayed using ELISA. The detection of increased levels of GLP-2 in a well containing a test compound identifies the test compound as a compound that can enhance GLP-2 secretions from L-cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com