Synthesis method of antibacterial agent cefoxitin acid
A technology for cefoxitin and antibacterial drugs, which is applied in the field of synthesis of antibacterial cefoxitin, can solve the problems of high cost, complicated production process, and low product yield of cefoxitin, and achieve easy implementation and shortened production The steps and synthesis process are simple and the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
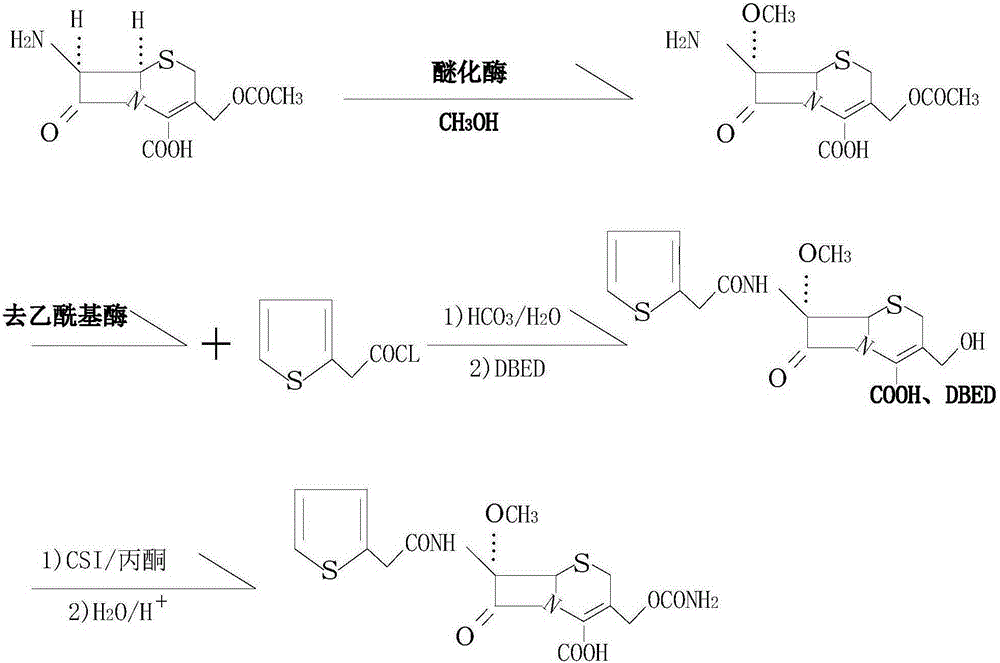
[0019] see figure 1 , in the embodiment of the present invention, a kind of synthetic method of antibacterial drug cefoxitin acid, use 7-amino cephalosporanic acid as raw material, use sodium bicarbonate solution to dissolve it, add etherification enzyme in the weak alkaline solution, and then Add methanol, introduce a methoxy group at the 7-position, and carry out an etherification reaction, wherein the molar ratio of methanol to 7-aminocephalosporanic acid is 1:1, the etherification reaction temperature is 30°C, and the etherification reaction time is 1h. After the conversion reaction, filter out the etherification enzyme; then add the deacetylase into the feed solution for hydrolysis, the hydrolysis temperature is 30°C, and the hydrolysis time is 0.5h. After the hydrolysis, add ethyl acetate to the solution and start Drop into 2-thiophene acetyl chloride reaction, the molar ratio of 2-thiophene acetyl chloride and 7-amino cephalosporanic acid is 1.2:1; After the reaction is...
Embodiment 2
[0023] In the embodiment of the present invention, a method for synthesizing the antibacterial drug cefoxitin acid uses 7-aminocephalosporanic acid as a raw material, dissolves it in a sodium bicarbonate solution, adds etherifying enzyme into the weakly alkaline solution, and then adds Methanol, introduce a methoxy group at the 7-position, carry out etherification reaction, wherein the molar ratio of methanol to 7-aminocephalosporanic acid is 2:1, the etherification reaction temperature is 25°C, the etherification reaction time is 2h, etherification After the reaction, filter out the etherification enzyme; then add the deacetylase to the feed solution for hydrolysis, the hydrolysis temperature is 25°C, and the hydrolysis time is 0.8h. After the hydrolysis, add ethyl acetate to the solution and start to drop Add 2-thiopheneacetyl chloride to react, the molar ratio of 2-thiopheneacetyl chloride to 7-aminocephalosporanic acid is 1.6:1; after the reaction is completed, drop benzath...
Embodiment 3
[0025] In the embodiment of the present invention, a method for synthesizing the antibacterial drug cefoxitin acid uses 7-aminocephalosporanic acid as a raw material, dissolves it in a sodium bicarbonate solution, adds etherifying enzyme into the weakly alkaline solution, and then adds Methanol, introduce a methoxyl group at the 7-position, and carry out an etherification reaction, wherein the molar ratio of methanol to 7-aminocephalosporanic acid is 1.5:1, the etherification reaction temperature is 20°C, and the etherification reaction time is 1.5h. After the conversion reaction, filter out the etherification enzyme; then add the deacetylase to the feed solution for hydrolysis, the hydrolysis temperature is 20°C, and the hydrolysis time is 0.7h. After the hydrolysis, add ethyl acetate to the solution and start Drop into 2-thiophene acetyl chloride reaction, the molar ratio of 2-thiophene acetyl chloride and 7-aminocephalosporanic acid is 1.4:1; Reaction finishes, drips benzath...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com