Pharmaceutical composition containing CpG oligonucleotide
A technology of oligonucleotides and compositions, applied in the field of pharmaceutical compositions, can solve the problems of inability to stimulate Th1 activity, inability to enhance cellular immunity, and hiding of hepatitis B virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
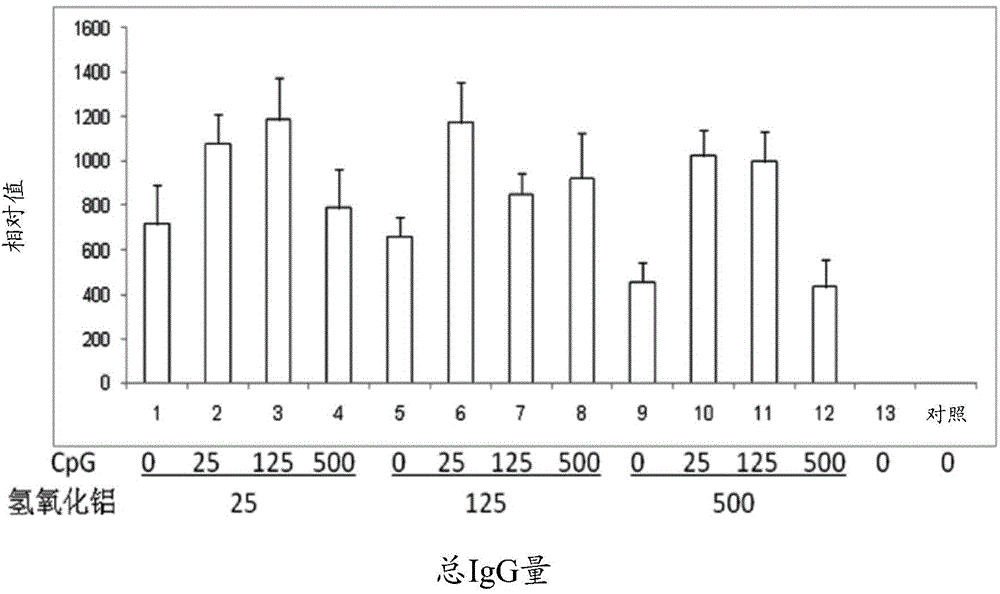
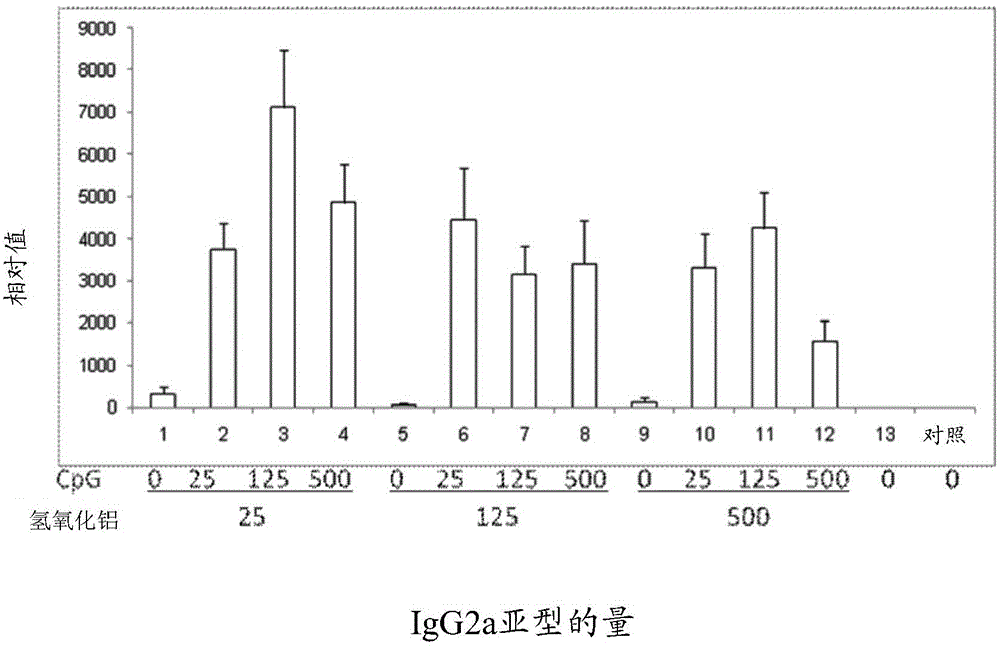
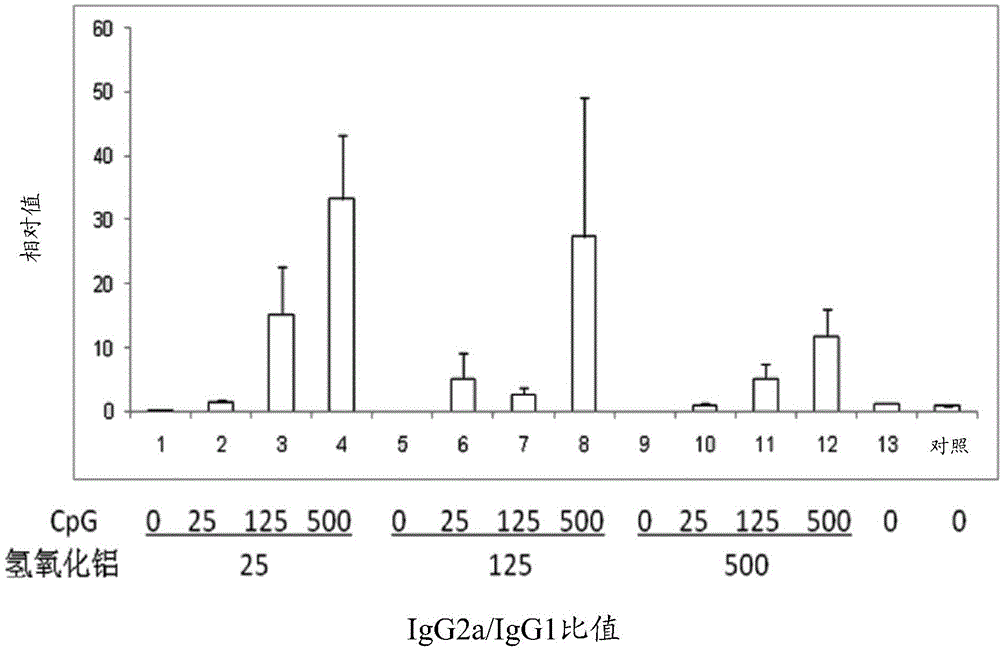
[0078] Example 1: Drugs comprising hepatitis B surface antigen (HBsAg) and aluminum hydroxide and CpG oligonucleotides combination
[0079] This embodiment provides a pharmaceutical composition comprising hepatitis B surface antigen (HBsAg), aluminum hydroxide and CpG oligonucleotides, HBsAg, Al(OH) in the pharmaceutical composition 3 and CpG-ODN concentrations are shown in Table 1 below.
[0080] Table 1
[0081] Sample serial number#
HBsAg (μg / ml)
Al(OH) 3 (μg / ml)
CpG (μg / ml)
1#
20
25
0
2#
20
25
25
3#
20
25
125
4#
20
25
500
5#
20
125
0
6#
20
125
25
7#
20
125
125
8#
20
125
500
9#
20
500
0
10#
20
500
25
11#
20
500
125
12#
20
500
500
[0082] 13#
PBS
PBS
PBS
[0083] Detect HBsAg, Al(OH) co...
Embodiment 2
[0103] Example 2: Pharmaceutical composition comprising hepatitis B surface antigen, aluminum hydroxide adjuvant and CpG oligonucleotide Induce antibody to produce immune response
[0104] This embodiment provides a pharmaceutical composition comprising hepatitis B surface antigen (HBsAg), aluminum hydroxide and CpG oligonucleotides, HBsAg, Al(OH) in the pharmaceutical composition 3 and CpG-ODN concentrations are shown in Table 2 below.
[0105] Table 2
[0106] Sample serial number#
HbsAg (μg / ml)
Al(OH) 3 (μg / ml)
CpG (μg / ml)
1#
20
300
125
2#
20
300
250
3#
20
300
500
4#
20
400
125
5#
20
400
250
6#
20
400
500
[0107] 7#
20
500
125
8#
20
500
250
9#
20
500
500
10#
20
500
PBS
[0108] Detect HBsAg, Al(OH) containing the different concentrations liste...
Embodiment 3
[0120] Example 3: Synergistic effect of aluminum hydroxide and CpG oligonucleotides at different concentrations
[0121] This embodiment provides a pharmaceutical composition comprising hepatitis B surface antigen (HBsAg), aluminum hydroxide and CpG oligonucleotides, HBsAg, Al(OH) in the pharmaceutical composition 3 and CpG-ODN concentrations are shown in Table 3 below.
[0122] table 3
[0123] Sample serial number#
HBsAg (μg / ml)
Al(OH) 3 (μg / ml)
CpG (μg / ml)
1#
20
300
125
2#
20
300
250
3#
20
300
500
4#
20
400
125
5#
20
400
250
6#
20
400
500
7#
20
500
125
8#
20
500
250
[0124] 9#
20
500
500
10#
20
500
PBS
[0125] Detect HBsAg, Al(OH) containing the different concentrations listed in Table 3 in the present embodiment according to the experimental...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com