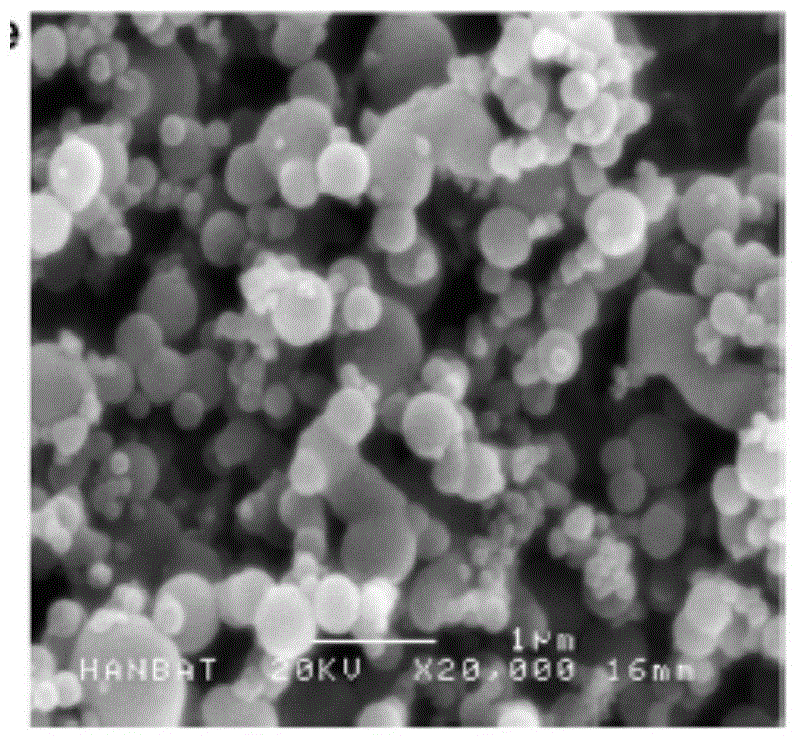
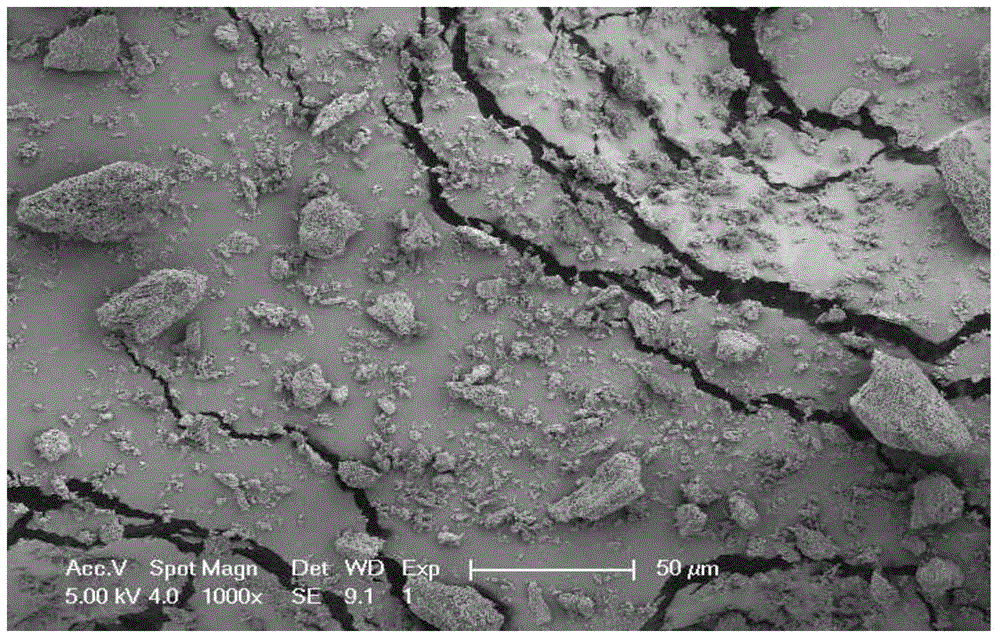
Ultrafine powder of HMG-CoA reductase inhibitor and preparation method of ultrafine powder
A reductase inhibitor and ultrafine powder technology, which is applied in the field of medicine, can solve the problems of supercritical fluid state temperature and pressure that have great influence, difficult configuration of production equipment, and large differences in product size, etc., and achieves good parallelism of production processes. , The powder morphology is stable and the uniformity is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] lovastatin raw material 5g, add 40ml methanol, heat to dissolve, add 15ml water, room temperature, 20kHz, 150W ultrasonic to obtain white crystals; collect, wash and dry to obtain ultrafine powder.
Embodiment 2
[0037] lovastatin raw material 150g, add 1200ml methanol, heat to dissolve, add 450ml water, room temperature, 30kHz, 2500W ultrasonic to obtain white crystals; collect, wash and dry to obtain ultrafine powder.
Embodiment 3
[0039] Add 10 g of atorvastatin raw material, add 30 ml of methanol, heat to dissolve, add 20 ml of water, cool in an ice bath, 20 kHz, 200 W ultrasound to obtain white crystals; collect, wash and dry to obtain ultrafine powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com