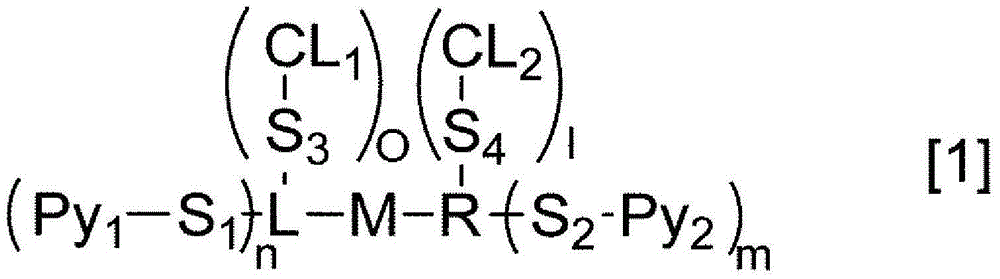Liquid crystal aligning agent containing crosslinkable compound having photoreactive group
A liquid crystal aligning agent and cross-linking technology, applied in the field of liquid crystal display elements, can solve the problems of low solubility of photopolymerizable compounds, precipitation of added amount, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0256] Although Examples and Comparative Examples are given below and the present invention will be described in more detail, the explanation of the present invention is not limited to these Examples. It should be noted that the abbreviations in the examples are as follows.
[0257]
[0258]
[0259] RM: 5,5-(biphenyl-4,4'-diylbis(oxygen))bis(pentane-5,1-diyl)bis(2-methacrylate)
[0260]
[0261] BODA: Bicyclo[3,3,0]octane-2,4,6,8-tetracarboxylic dianhydride
[0262] CBDA: 1,2,3,4-cyclobutanetetracarboxylic dianhydride
[0263] TCA: 2,3,5-Tricarboxycyclopentylacetic dianhydride
[0264]
[0265] PCH: 1,3-diamino-3-[4-(4-heptylcyclohexyl)phenoxy]benzene
[0266] DA-Col: Cholesteryl 3,5-diaminobenzoate
[0267] DBA: 3,5-diaminobenzoic acid
[0268] DA-1: 2-(methacryloyloxy)ethyl-3,5-diaminobenzoate
[0269] DA-2: N 1 ,N 1 -Diarylbenzene-1,2,4-triamine
[0270]
[0271] 3-AMP: 3-pyridylmethylamine
[0272] (NMP: N-methyl-2-pyrrolidone
[0273] BCS: Butyl...
Synthetic example 1
[0283] (Synthesis Example 1) Synthesis of CL-1
[0284] (Step 1) Synthesis of CL-1-1, the precursor of CL-1
[0285]
[0286] Add 3,3',5,5'-tetrakis(methoxymethyl)-[1,1'-biphenyl]-4,4'-diol 10.9g, DMF200mL, 6 12.3 g of chloro-1-hexanol, 24.9 g of potassium carbonate, and 2.5 g of potassium iodide were stirred while heating to 100°C. After the reaction, the reaction system was poured into 1 L of water, neutralized with 1N-hydrochloric acid (HCl) aqueous solution, and the precipitate was filtered. This filtrate was dried to obtain 16.9 g of CL-1-1 (yellow solid) (99% yield).
[0287] (Step 2) Synthesis of CL-1 from precursors
[0288]
[0289] CL-1-116.9 g, 7.30 g of triethylamine, and 160 mL of THF were added to a 300 mL four-necked flask. The inside of the system was cooled to 0° C., 7.30 g of methacryloyl chloride was added, and stirred at room temperature (rt: 25° C.). After completion of the reaction, the reaction system was poured into 500 mL of water, and extra...
Synthetic example 2
[0292] (Synthesis Example 2) Synthesis of CL-2
[0293] (Step 1) Synthesis of CL-2-1, the precursor of CL-2
[0294]
[0295] 17.8 g of trans-methyl p-coumarate, 250 mL of DMF, 20.5 g of 6-chloro-1-hexanol, 41.5 g of potassium carbonate, and 1.7 g of potassium iodide were added to a 500 mL three-necked flask, and stirred at 100°C. After the reaction, the reaction system was poured into 1.2 L of water, neutralized with 1N-hydrochloric acid (HCl) aqueous solution, and the precipitate was filtered. The filtrate was dissolved in 300 mL of ethyl acetate, extracted with saturated brine, dehydrated and dried by adding anhydrous magnesium sulfate to the organic layer, filtered, and the solvent was distilled off using a rotary evaporator to obtain 25.6 g of the target compound CL -2-1 (white solid) (92% yield).
[0296] (Step 2) Synthesis of CL-2-2, the precursor of CL-2
[0297]
[0298] 25.6 g of CL-2-1, 200 mL of THF, and 11.2 g of triethylamine were added to a 500 mL three-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com