Cefdinir dispersible tablet and preparation method thereof
A technology for cefdinir and dispersible tablets, applied in the field of cefdinir dispersible tablets and their preparation, can solve the problems of poor fluidity, poor water solubility, instability and the like, and achieves simplified process operation, improved product yield, guaranteed yield and The effect of cost control
Inactive Publication Date: 2015-12-23
SICHUAN SAIZHUO PHARMACY
View PDF5 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the cefdinir bulk drug has poor water solubility, is prone to static electricity, and has poor fluidity. It is unstable to conditions such as humidity, heat, and light, and the impurity content seriously affects the safety and effectiveness of the product.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0040]Embodiment 1 (in this embodiment, getting 1 weight part is 1g)
[0041]
Embodiment 2
[0042] Embodiment 2 (in this embodiment, getting 1 weight part is 1g)
[0043]
Embodiment 3
[0044] Embodiment 3 (in this embodiment, getting 1 weight part is 1g)
[0045]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Login to View More
Abstract
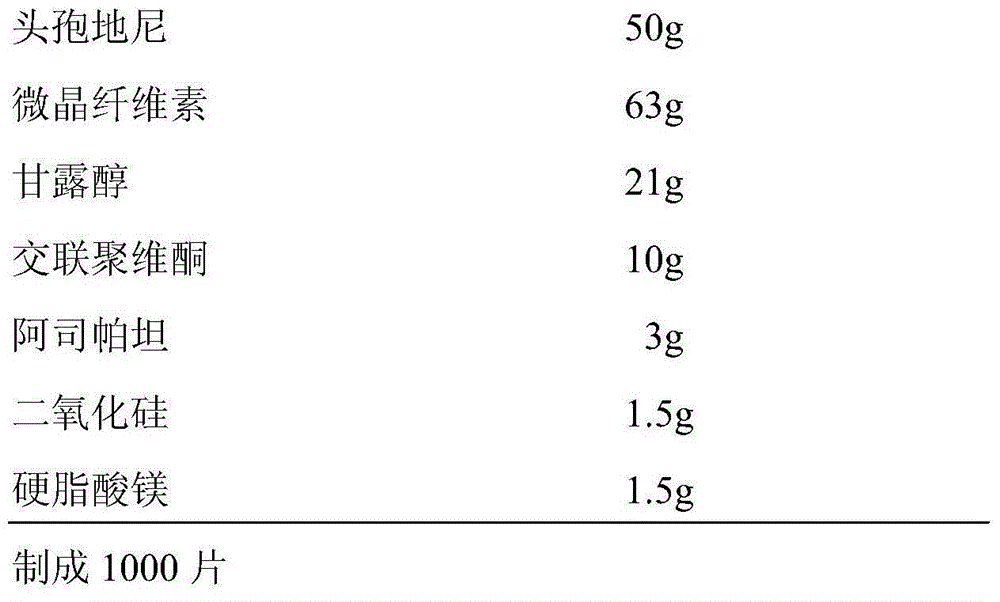
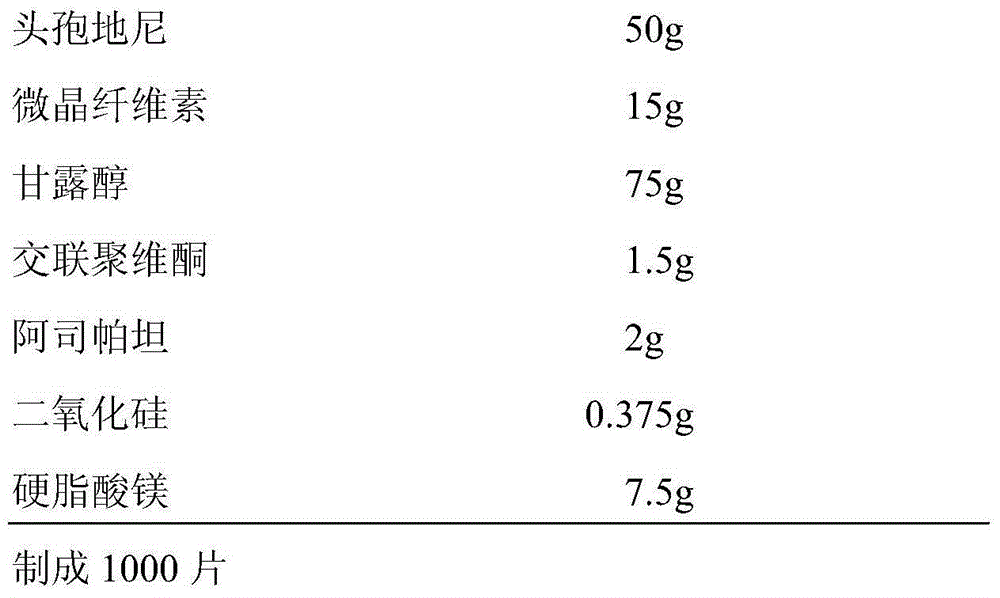
The invention discloses a cefdinir dispersible tablet and a preparation method thereof. The cefdinir dispersible tablet is prepared from, by weight, 50 parts of cefdinir, 15-75 parts of microcrystalline cellulose, 15-75 parts of mannitol, 1.5-12 parts of polyvinylpolypyrrolidone, 1.5-15 parts of aspartame, 0.375-7.5 parts of silicon dioxide and 0.375-7.5 parts of magnesium stearate. The formula of the cefdinir dispersible tablet is optimized, the raw materials are prevented from being micronized in the direct powder compression preparation process, and process operation is simplified; meanwhile, cefdinir and auxiliary materials do not need to be separately screened, the yield of products is increased, the cefdinir dispersible tablet is suitable for expanded production, after expanded production, loose tablets, cracking tablets, sticking and the like are avoided, the dissolution rate of prepared medicine is high, the impurity content is low, the tablet difference is low, the yield is high, and the quality stability and yield of the medicine are guaranteed.
Description
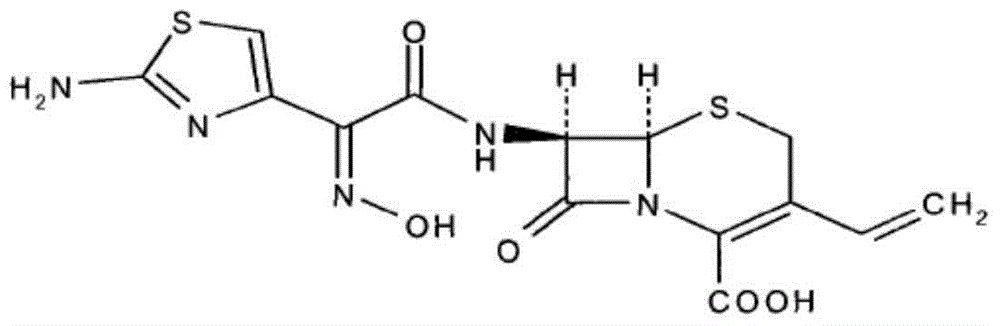
technical field [0001] The invention belongs to the field of pharmaceutical preparations, in particular to a cefdinir dispersible tablet and a preparation method thereof. Background technique [0002] The chemical name of cefdinir (cefdinir, CASNO.: 91832-40-5) is: (6R, 7R)-7-[(Z)-2-(2-amino-4thiazolyl)-2-hydroxyimino -Acetamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. [0003] Its structural formula is: [0004] [0005] Cefdinir is an oral third-generation cephalosporin, which has the advantages of broad antibacterial spectrum and low toxicity. It can be used to treat tonsillitis, sinusitis, otitis media, acute bronchitis, pneumonia, abdominal cavity, and urogenital tract infection. It is widely used in infectious diseases caused by sensitive bacteria such as internal medicine, surgery, dermatology, obstetrics and gynecology, and urology. [0006] The existing application numbers are CN201410220481.7, CN201310194902.9, CN201310470821.7 an...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/20A61K31/546A61P31/04A61P11/04A61P11/02A61P27/16A61P11/00A61P1/00A61P13/00A61P15/00
Inventor 罗厚华向进袁德彬李亚真鲁韬
Owner SICHUAN SAIZHUO PHARMACY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com