A kind of benzylation method of monosaccharide methyl glycoside
A monosaccharide methyl glycoside and benzylation technology, applied in the chemical field, can solve the problems of unstable solvent, increase of by-product toluene, low boiling point of solvent, etc., and achieve the effect of simplifying post-processing operation, reducing sodium hydride, and suitable boiling point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
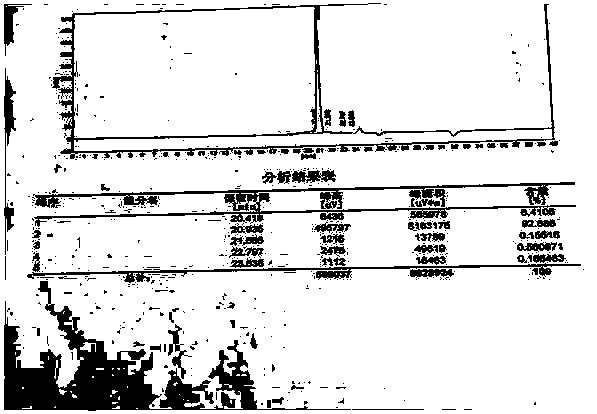
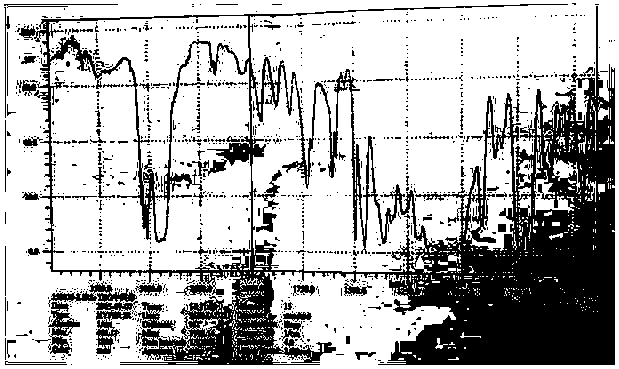
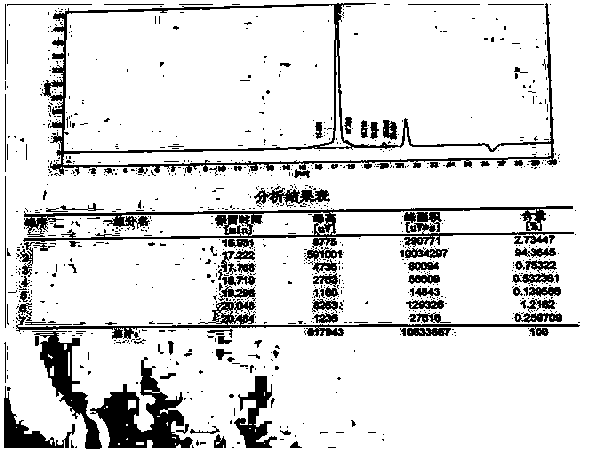
Image
Examples
Embodiment 1
[0040] Methyl-2,3,4,6-tetra-O-benzyl-α-D-glucopyranoside
[0041] In a 250ml three-neck flask equipped with a stirrer, a thermometer, and a condenser tube, add methyl-α-D-glucopyranoside 9.7g (50.0mmol) and sodium hydride paraffin oil dispersion (sodium hydride content 55wt%) successively 9.16g (209.9mmol), 60ml of toluene, 27.85g (220.0mmol) of benzyl chloride, heated to about 105-110°C, tracked by liquid chromatography, and kept warm until the reaction was complete. After the heat preservation reaction is completed, cool down to about 60°C, slowly add water dropwise, and react with the residual sodium hydride until no bubbles are formed. The solid matter was removed by filtration, and the filter cake was washed with 15 ml x 2 times of toluene, and the organic layers were combined. The organic layer was heated to 65-70° C., washed with water until neutral, and washed with 20 ml of water each time, and washed 3-4 times (the first washing water was discarded, and the other was...
Embodiment 2
[0043] Methyl-2,3,4,6-tetra-O-benzyl-α-D-glucopyranoside
[0044] In a 250ml three-necked flask equipped with a stirrer, a thermometer, and a condenser tube, add methyl-α-D-glucopyranoside 9.7g (50.0mmol) and sodium hydride paraffin oil dispersion (sodium hydride content 55%) in sequence 9.16g (209.9mmol), 60ml of toluene, 26.56g (210.0mmol) of benzyl chloride, heated to about 90-95°C, tracked by liquid chromatography, and kept warm until the reaction was complete. After the heat preservation reaction is completed, cool down to about 60°C, slowly add water dropwise, and react with the residual sodium hydride until no bubbles are formed. The solid matter was removed by filtration, and the filter cake was washed with 15 ml x 2 times of toluene, and the organic layers were combined. The organic layer was heated to 65-70° C., washed with water until neutral, and washed with 20 ml of water each time, and washed 3-4 times (the first washing water was discarded, and the other washin...
Embodiment 3
[0046] Methyl-2,3,4,6-tetra-O-benzyl-α-D-galactopyranoside
[0047] In a 250ml three-neck flask equipped with a stirrer, a thermometer, and a reflux separator (equipped with a condenser tube), 10.6g (50.0mmol) of methyl-α-D-galactopyranoside monohydrate, toluene 100ml, heat up to 90-95°C, control the vacuum degree -0.03--0.04Mpa, reflux and divide water to remove the crystal water in methyl-α-D-galactopyranoside monohydrate. After dehydration is completed, cool down to 35-40°C, and change the reflux water separator to a reflux condensing device. Add sodium hydride paraffin oil dispersion (content 55%) 10.0g (229.2mmol) and benzyl chloride 30.5g (240.9mmol) successively, heat up to about 90-95°C, follow by liquid chromatography, keep warm until the reaction is complete. After the heat preservation reaction is completed, cool down to about 60°C, slowly add water dropwise, and react with the residual sodium hydride until no bubbles are formed. The solid matter was removed by fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com