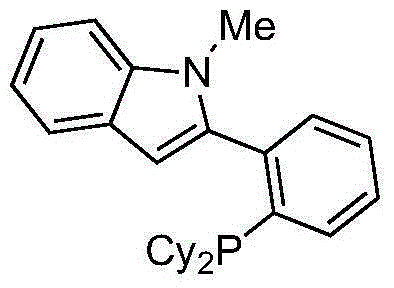
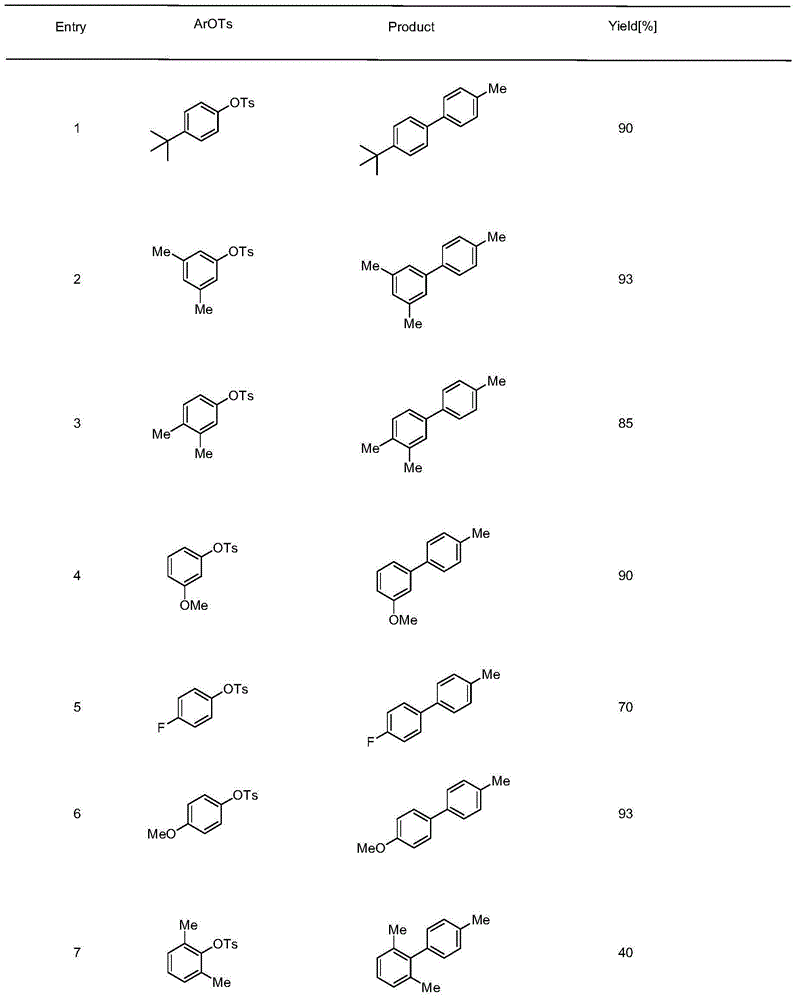
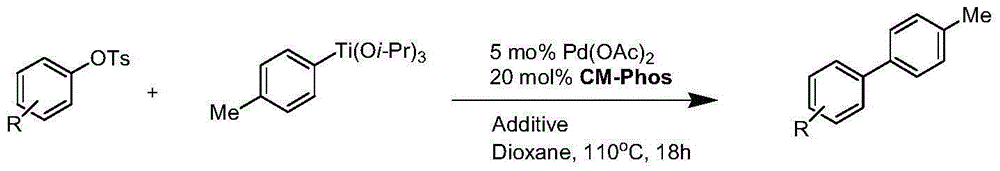Cross coupling reaction of arenesulphonate substrate and organic titanium
A cross-coupling reaction, arylsulfonic acid technology, applied in the preparation of organic compounds, organic substitution, organic chemistry, etc., can solve the problem of low activity of arylsulfonic acid esters, and achieve easy storage and large-scale synthesis. , the effect of reducing the reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment one: the coupling reaction of aryl p-toluenesulfonate and 4-methylphenisopropoxide titanium
[0016] In a 20mL Schlenk tube, add palladium acetate (0.025mmol) and phosphine ligand (palladium: phosphine ligand ratio is 5mol%: 20mol%), then add a magnetic stirring bar equipped with a polytetrafluoroethylene coating, and the system is replaced by Under nitrogen protection, 0.1 mL of freshly distilled triethylamine and 1.0 mL of dichloromethane were added, stirred and heated gently to a slight boil, and then sucked dry under reduced pressure to form a palladium complex.
[0017] Aryl p-toluenesulfonate (0.5 mmol), titanium 4-methylphenisopropoxide (2.5 mmol) and potassium phosphate (0.125 mmol) were then added under nitrogen. Finally, 1,4-dioxane solution (2.0 mL) was added, and then the Schlenk tube was placed in a preheated oil bath at 110° C. for 18 to 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, about 10 mL of...
Embodiment 2
[0021] Embodiment two: the coupling reaction of aryl p-toluenesulfonate and 4-methoxyphenylisopropoxide titanium
[0022] In a 20mL Schlenk tube, add palladium acetate (0.025mmol) and phosphine ligand (palladium: phosphine ligand ratio is 5mol%: 20mol%), then add a magnetic stirring bar equipped with a polytetrafluoroethylene coating, and the system is replaced by Under nitrogen protection, 0.1 mL of freshly distilled triethylamine and 1.0 mL of dichloromethane were added, stirred and heated gently to a slight boil, and then sucked dry under reduced pressure to form a palladium complex.
[0023] Aryl p-toluenesulfonate (0.5 mmol), titanium 4-methoxyphenisopropoxide (2.5 mmol) and potassium phosphate (0.125 mmol) were then added under nitrogen. Finally, 1,4-dioxane solution (2.0 mL) was added, and then the Schlenk tube was placed in a preheated oil bath at 110° C. for 18 to 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, about 10 m...
Embodiment 3
[0027] Embodiment three: the coupling reaction of heterocyclic aryl p-toluenesulfonate and organic titanium
[0028] In a 20mL Schlenk tube, add palladium acetate (0.025mmol) and phosphine ligand (palladium: phosphine ligand ratio is 5mol%: 20mol%), then add a magnetic stirring bar equipped with a polytetrafluoroethylene coating, and the system is replaced by Under nitrogen protection, 0.1 mL of freshly distilled triethylamine and 1.0 mL of dichloromethane were added, stirred and heated gently to a slight boil, and then sucked dry under reduced pressure to form a palladium complex.
[0029] Heterocyclic aryl p-toluenesulfonate (0.5 mmol), organotitanium (2.5 mmol) and potassium phosphate (0.125 mmol) were subsequently added under nitrogen. Finally, 1,4-dioxane solution (2.0 mL) was added, and then the Schlenk tube was placed in a preheated oil bath at 110° C. for 18 to 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, about 10 mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com