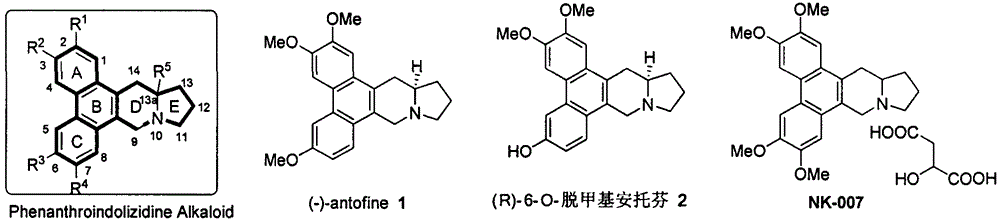
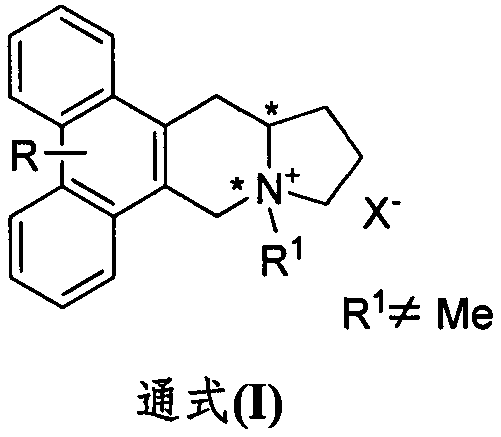
Phenanthroindolizidine alkaloid quaternary ammonium salt derivative and preparation and plant virus resisting application thereof
A technology of phenanthroindolizidine and anti-plant virus agent, which is applied in the fields of phenanthroindolizidine alkaloid quaternary ammonium salt derivatives and their preparation and anti-plant virus applications, and can solve problems such as lack of reports, Achieve the effect of good in vivo activity, high in vitro anti-TMV activity, and excellent anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Synthesis of phenanthrene and indolizidine alkaloid quaternization product
[0027]
[0028] Dissolve R-serphine or S-serphine (590 mg, 1.5 mmol) in CHCl 3 , then add iodomethane or bromide (3-propene bromide, 3-bromopropyne, 1-bromo-2-butyne, bromoacetonitrile, bromoacetamide, 2-bromo-N-methylacetamide, 2 -Bromo-N,N-dimethylacetamide, benzyl bromide, p-trifluoromethylbenzyl bromide, p-trifluoromethylbenzyl bromide, 2-bromoacetophenone) (3 mmol). The reaction was heated to reflux for 24-48h, and the reaction was desolvated under reduced pressure to obtain a crude product, which was then separated by normal pressure column chromatography (CH 2 Cl 2 :MeOH=20:1) to obtain the target product.
[0029] Compound I-S-1a
[0030] Pale yellow solid, Mp: 216-217°C. Yield: 20%. 1 HNMR (400MHz, DMSO-d 6)δ8.12(s, 1H), 8.10(s, 1H), 7.47(s, 1H), 7.39(s, 1H), 5.20(d, J=16.0Hz, 1H), 4.97(d, J=16.0 Hz, 1H), 4.52-4.39(m, 2H), 4.30(br, 1H), 4.07(s, 6H), 4.00(s, 3H),...
Embodiment 2
[0093] Example 2: Research on the physicochemical properties of phenanthrene and indolizidine alkaloid quaternary ammonium salt derivatives
[0094] Above-mentioned preferred phenanthrene and indolizidine alkaloid quaternary ammonium derivatives I-S-1a, I-S-1b, I-R-1a, I-R-1b, I-R-2a, I-S-7b, I-S-8a, I-S-8b, I-R- 8a, I-S-10a, I-S-10b, I-S-11a, I-S-11b, I-S-12 compared with their respective control samples (R) / (S)-siraphyrine, characterized by preferred compounds compared to known compounds Compared with it, it has outstanding advantages, specifically: (1) the chemical stability is significantly enhanced, and the deterioration speed is obviously slower than them when placed at room temperature under the same conditions or exposed to sunlight for the same time; (2) the water solubility is significantly enhanced, (R) / ( S)-sermenine is insoluble in water, and the water solubility of the above quaternary ammonium salt derivatives is greater than 5mg / mL; the above two points have a ...
Embodiment 3
[0095] Embodiment 3: the assay of anti-tobacco mosaic virus activity, assay procedure is as follows:
[0096] 1. Virus purification and concentration determination:
[0097] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0098] 2. Compound solution preparation:
[0099] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL mother solution, and then diluted with 1‰ Tween 80 aqueous solution to the required concentration; Ningnanmycin preparation was directly diluted with water.
[0100] 3. In vitro effect:
[0101] Rub inoculation of leaves of Shanxi tobacco at the right age, rinse with running water, the virus concentration is 10 μg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com