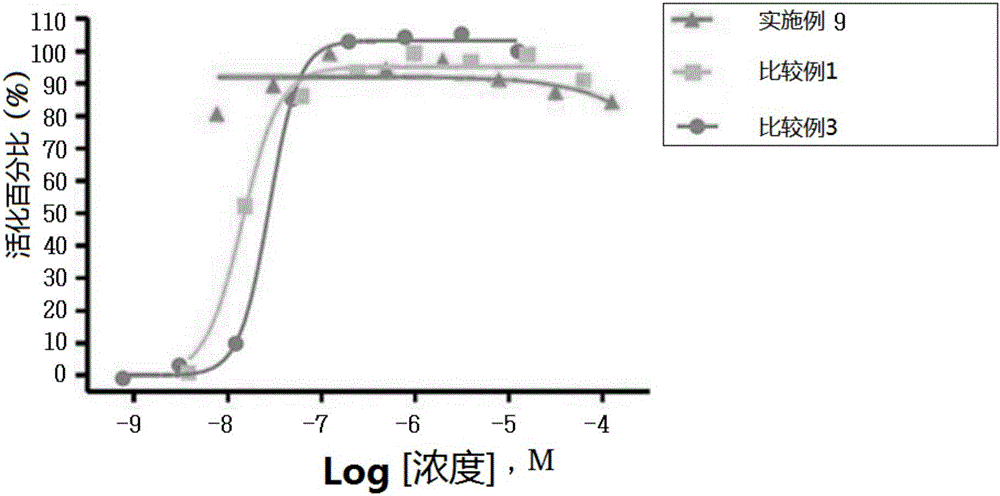
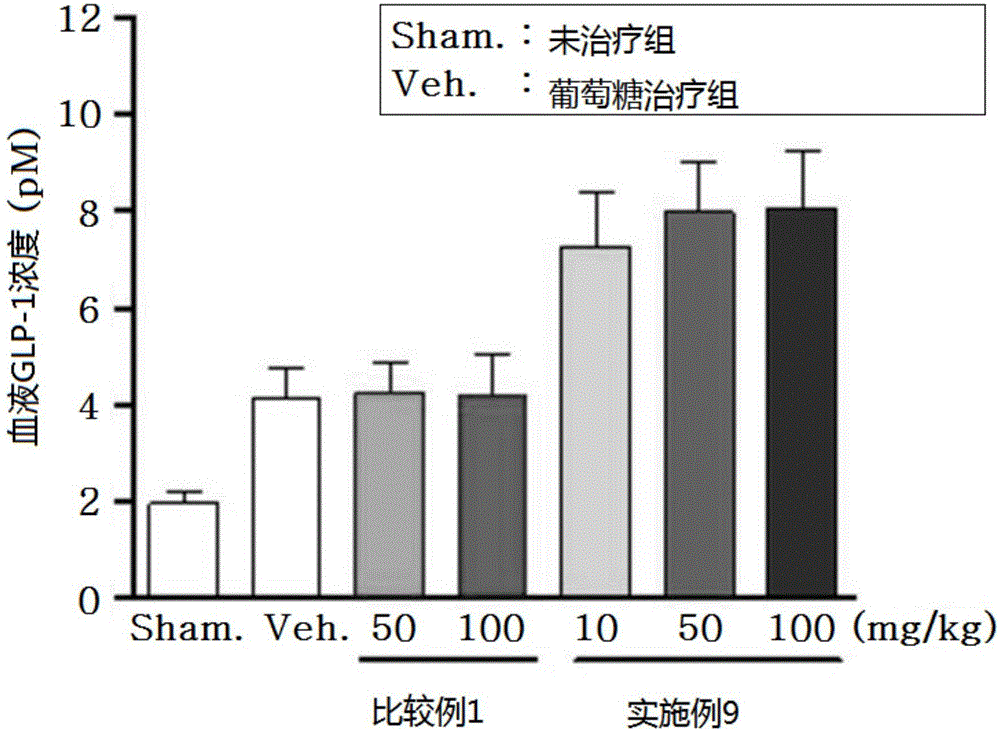
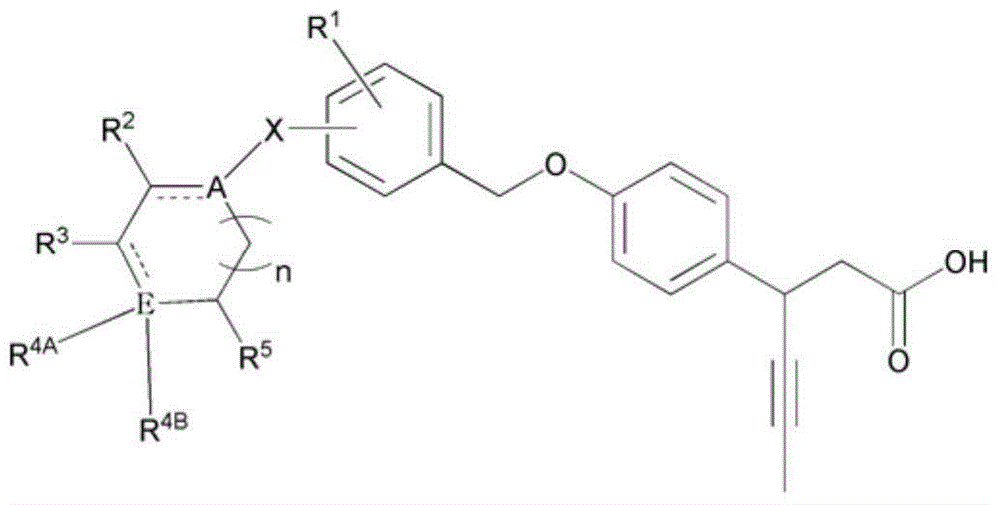
Novel 3-(4-(benzyloxy)phenyl)hex-4-inoic acid derivative, method of preparing same and pharmaceutical composition for preventing and treating metabolic disease including same as effective ingredient
一种苄氧基、苯基的技术,应用在3-苯基)己-4-炔酸衍生物领域,能够解决增加体重、失去治疗药物反应性、胃肠道问题等问题,达到促进活化的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0165] In the preparation method of the compound represented by Formula 1 of the present invention, step 1) is to prepare the compound represented by Formula 4 by causing a coupling reaction between the compound represented by Formula 2 and the compound represented by Formula 3. More specifically, the compound represented by Formula 2, the compound represented by Formula 3, and triphenylphosphine were all mixed to obtain a mixed solution. Azocarboxylate reagent (Azocarboxylate reagent) is slowly added to the mixed solution at a temperature of -5°C to 10°C, followed by causing Mitsunobureaction to obtain the compound represented by Formula 4.
[0166] At this time, the azodicarboxylate reagent can be selected from the group consisting of diethyl azodicarboxylate (DEAD) and diisopropyl azodicarboxylate (DIAD), and preferably diethyl azodicarboxylate Isopropyl ester (DIAD).
[0167] The reaction solvent here may be selected from the group consisting of tetrahydrofuran (THF), dic...
manufacture example 1
[0254] Production Example 1: Preparation of ethyl 3-(4-hydroxyphenyl)hex-4-ynoate
[0255]
[0256] 3-(4-Hydroxyphenyl)-hex-4-ynoic acid (20.0 g) and ethanol (200 mL) were charged into a 250 mL flask under a nitrogen atmosphere, followed by stirring to dissolve them. Sulfuric acid (9.6 mL) was slowly added to this at room temperature. The mixture was stirred at reflux for at least 6 hours. Upon completion of the reaction, distilled water (150 mL) was slowly added thereto, followed by extraction with ethyl acetate (200 mL). The extracted organic layer was dried under reduced pressure to obtain the target compound (19.5 g, 85.7%).
[0257] 1 HNMR (400MHz, CDCl 3 ): δ7.25(2H,d), 6.78(2H,d), 4.95(1H,s), 4.14(2H,m), 4.04(1H,m), 2.68(2H,m), 1.84(3H, d), 1.29(3H,t).
manufacture example 2
[0258] Production Example 2: Preparation of (S)-3-(4-hydroxyphenyl)hex-4-ynoic acid ethyl ester
[0259]
[0260] (S)-3-(4-Hydroxyphenyl)-hex-4-ynoic acid (20.0 g) and ethanol (200 mL) were charged into a 250 mL flask under a nitrogen atmosphere, followed by stirring to dissolve them. Sulfuric acid (9.6 mL) was slowly added to this at room temperature. The mixture was stirred at reflux for at least 6 hours. Upon completion of the reaction, distilled water (150 mL) was slowly added thereto, followed by extraction with ethyl acetate (200 mL). The extracted organic layer was dried under reduced pressure to obtain the target compound (21.2 g, 93.2%).
[0261] 1 HNMR (400MHz, CDCl 3 ): δ7.25(2H,d), 6.78(2H,d), 4.95(1H,s), 4.14(2H,m), 4.04(1H,m), 2.68(2H,m), 1.84(3H, d), 1.29(3H,t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com