Method for determining substitution degree of hydroxypropyl chitosan
A technology of hydroxypropyl chitosan and chitosan, which is applied in the direction of color/spectral characteristic measurement, etc., can solve problems such as difficulty in determining the degree of substitution of hydroxypropyl chitosan, and unseen problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
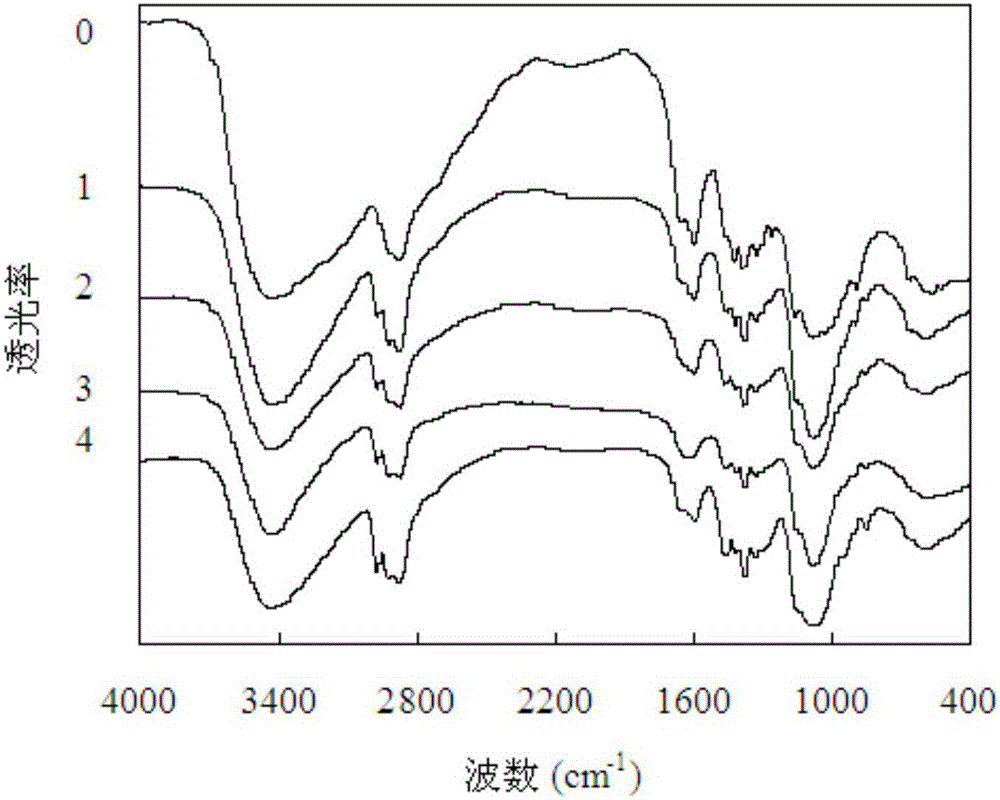
[0035] Reagents: KBr is optically pure, and acetanilide is a standard substance, both from Sinopharm Reagent Co., Ltd. Chitosan, derived from Laixi Haili Bioengineering Co., Ltd.; hydroxypropyl chitosan, using Chitosan No. 0 as raw material, reacts differently with propylene oxide at 45-60°C under alkaline conditions Time, hydroxypropyl chitosan No. 1-No. 4 with different degrees of substitution purified by dealkalization.
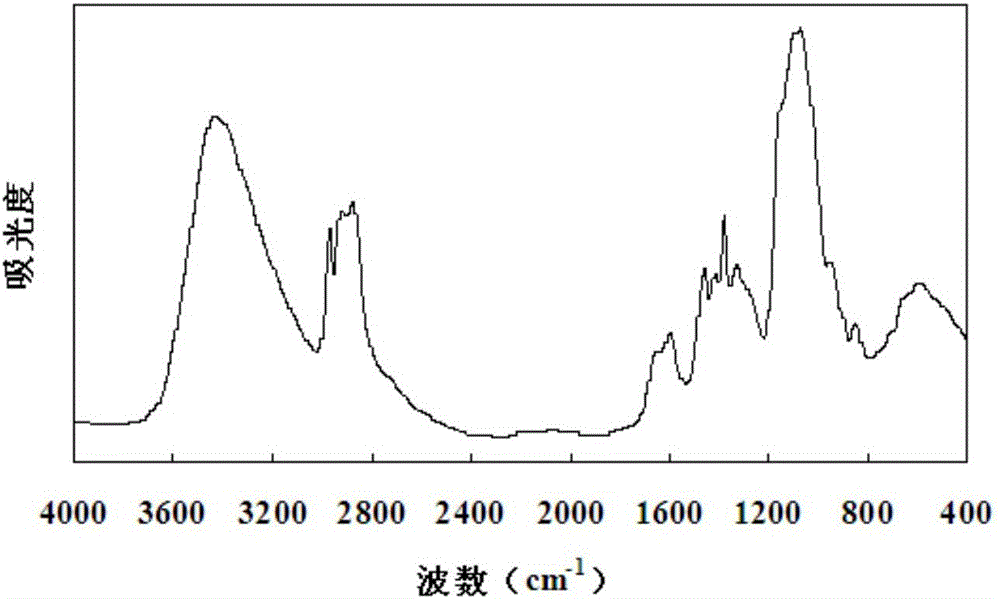
[0036] Instrument: the present invention is applicable to various Fourier transform infrared spectrometers, and instrument used in the present invention is the Fourier transform infrared spectrometer TENSOR27 of German Bruker (Bruker) company; The degree of acetylation and the degree of substitution of hydroxypropyl chitosan were completed in the testing center (national open laboratory) of the Institute of Bioenergy of the Chinese Academy of Sciences certified by the National Bureau of Metrology, and the instrument used was the VarioELcube elemental analy...
Embodiment 2
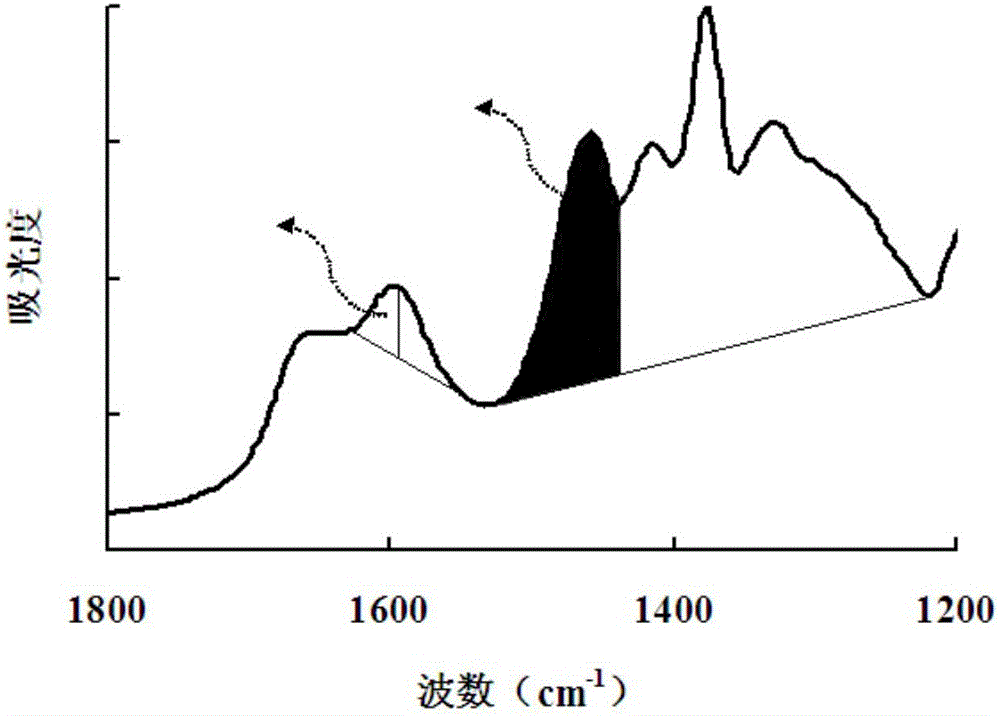
[0052] The hydroxypropyl chitosan samples A, B, C, D, E, and F to be tested were all purified by hydroxypropylation and dealkalization. The degree of deacetylation of the raw material chitosan is 92.2%, 93.8%, 94.4%, 95.1%, 96.3%, 97.6%. Compared with the raw chitosan (95.1%) of the hydroxypropyl chitosan sample used to establish the linear regression equation, the deacetylation degree differs by up to 3.05%. The hydroxypropyl chitosan to be tested dried according to the method (1) is passed through the Fourier transform infrared spectrometer according to the step (2a) and background detection and background processing are performed, so as to obtain an infrared absorption spectrum. According to the method of step (2)b, in the OPUS software, select the C baseline integration method to measure the methyl characteristic peak 1460-1456cm -1 The absorbance value A1 of the peak height, with the whole waveform 1530-1215cm -1 The line connecting the troughs is the baseline BL1, and ...
Embodiment 3
[0056] The dealkalization purification method of hydroxypropyl chitosan
[0057] After the raw material chitosan undergoes hydroxypropylation reaction with propylene oxide under alkaline conditions, it needs to be purified by dealkalization. The de-alkalization purification method of the sample includes the de-alkalization method of alcohol washing, ketone precipitation, dialysis and partial neutralization ultrafiltration de-alkalization method.
[0058] In the alcohol washing ketone precipitation dialysis dealkalization method, firstly, the product mixture is suction-filtered to remove the solvent and residual alkali. Add water to dissolve the crude product after suction filtration, filter, add ethanol to the filtrate to make it contain 75% ethanol, add acetone while stirring to precipitate the swollen chitosan, filter, and add the precipitate to 75% ethanol , and so on, until the ethanol aqueous solution of hydroxypropyl chitosan is neutral, then precipitate it with acetone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com