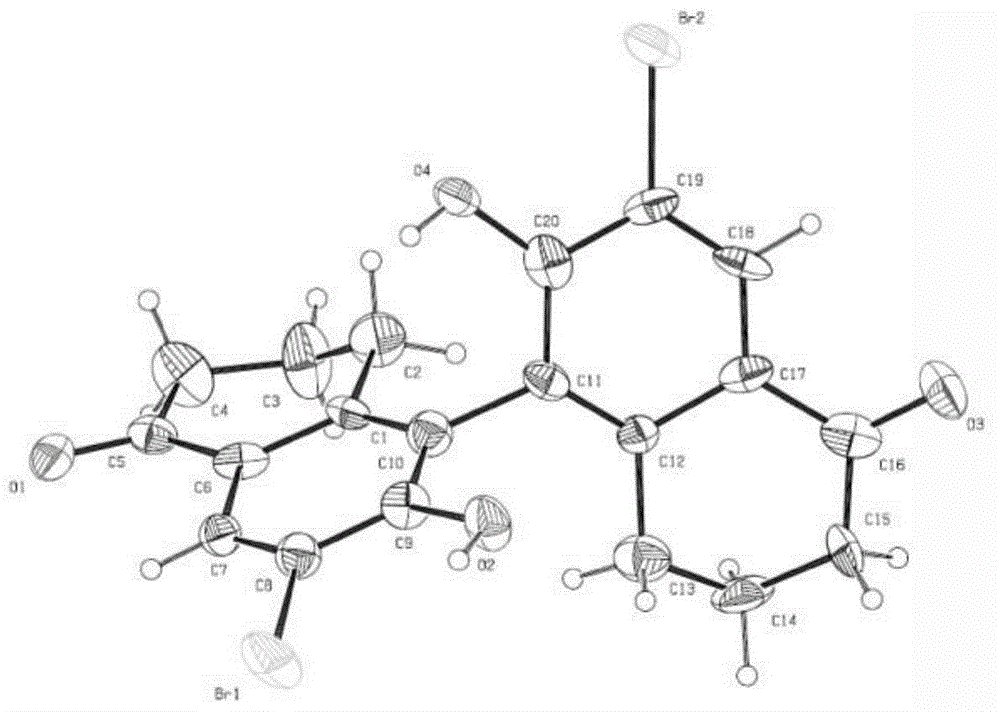
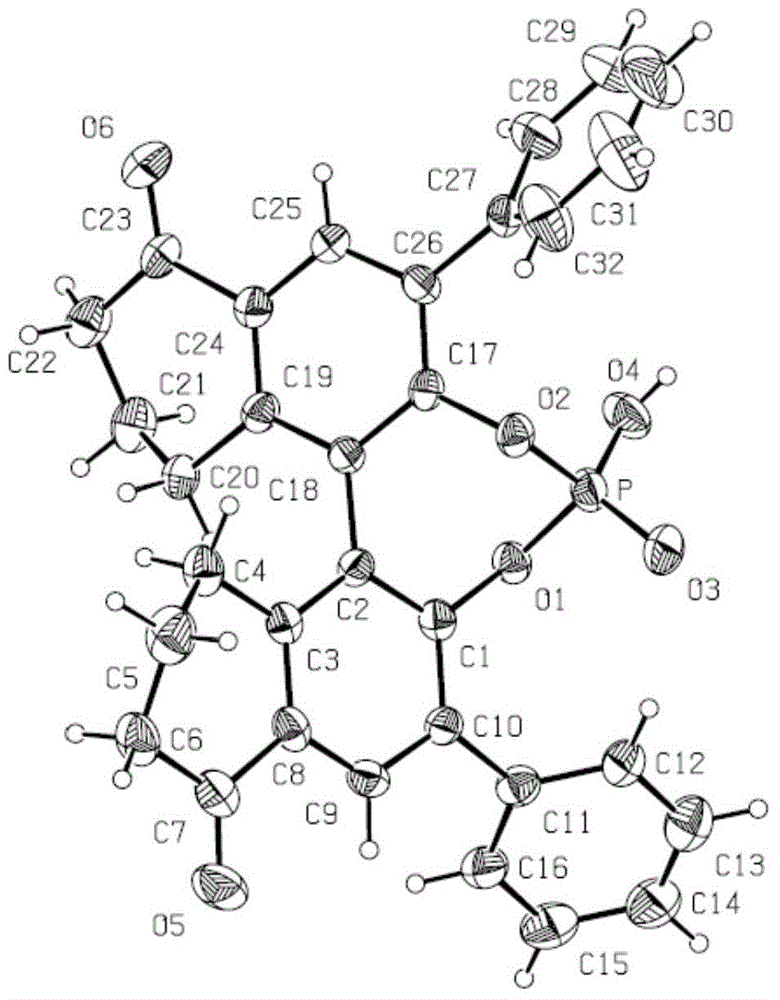
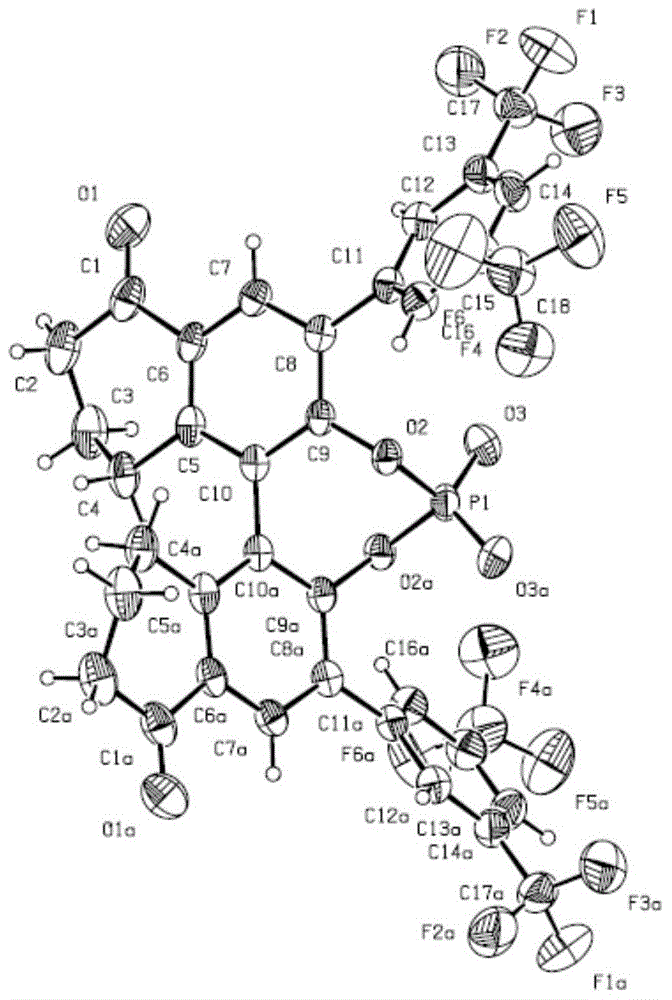
Chiral phosphoric acid with 5,5'-bitetralone skeleton and preparation method thereof
A technology of tetralone and phosphoric acid, which is applied in the field of intermediates involved in the preparation process, can solve the problems of low activity of organic chiral phosphoric acid catalysts, and achieve the effects of short steps, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the preparation of (R)-5,5'-tetralone-6,6'-diol (2)
[0051]
[0052] The reaction equation is shown in the above formula, wherein, DDQ is 2,3-dichloro-5,6-dicyano-1,4-benzoquinone;
[0053] Weigh (R)-H into a 500mL single-necked bottle 8 -BINOL (4.42g, 15.0mmol), add 100mL 1,4-dioxane and 5mLH 2 O stirred to dissolve. DDQ (13.62 g, 60.0 mmol) was added under ice cooling. After reacting at 25°C for 4 hours, filter with suction, and wash the filter residue with 50 mL of 1,4-dioxane. Concentrate the filtrate, add 200mL ethyl acetate, saturated Na 2 SO 3 The solution was washed three times. Anhydrous Na 2 SO 4 The organic phase was dried, filtered with suction, concentrated, and directly cast into the next step. It can also be purified by silica gel column chromatography (petroleum ether: ethyl acetate, volume ratio 2:1 ~ 1:2) to obtain a white solid. Melting point 263~264℃; [α] D 20 =+129.2 (c0.48, MeOH); IR (film) 3164, 2945, 1648, 1571, 1274...
Embodiment 2
[0054] Example 2, the preparation of (R)-7,7'-dibromo-5,5'-tetralone-6,6'-diol (3)
[0055]
[0056] In a water bath at 25°C, add 100 mL of 1,4-dioxane to a 250 mL single-necked bottle, add HBr (48% aq) (7.5 mL, 66.0 mmol) under stirring, and then add H 2 o 2 (35% aq) (3.7 mL, 42.9 mmol), and the product from the previous step was added. After reacting for 1 hour, add 0.95g Na 2 SO 3 Dissolve the solution in 25 mL of water, stir for half an hour, adjust the pH to 8-9 with 10% NaOH aqueous solution, and wash with ethyl acetate three times. Use 6mol / L dilute hydrochloric acid to acidify the aqueous phase to pH 1~2, extract with ethyl acetate, anhydrous Na 2 SO 4 Drying, suction filtration, concentration, and silica gel column chromatography (petroleum ether:ethyl acetate, volume ratio 3:1-3:2) yielded 4.90 g of a light yellow solid product, and the total yield of the two steps was 68%. Melting point 280~281℃; [α] D 20 =+83.0(c0.42, MeOH); IR(film) 3281, 2947, 1662, 15...
Embodiment 3
[0057] Example 3, the preparation of (R)-7,7'-diphenyl-5,5'-bitetralone-6,6'-diol (4)
[0058]
[0059] The reaction equation is shown in the above formula, wherein Ac is acetyl; Ph is phenyl; n Bu is n-butyl; Ad is adamantyl; DME is ethylene glycol dimethyl ether;
[0060] Under argon protection, compound (R)-3 (5.0g, 10.46mmol), phenylboronic acid (4.46g, 36.61mmol), palladium acetate (0.0472g, 0.21mmol), bis(adamantyl ) n-butylphosphine (0.094g, 0.262mmol), 90mL ethylene glycol dimethyl ether and 45mL 1.0mol / L potassium carbonate solution. The reactor was placed in a 95°C oil bath and stirred. Stop heating after 11 hours, cool to room temperature, add 50 mL of saturated ammonium chloride solution, acidify to pH 3-4 with 1.6 mol / L dilute hydrochloric acid in ice bath, let stand for 2 hours, and suction filter (obtain filtrate 1). The filter cake was dissolved in dichloromethane / methanol, anhydrous Na 2 SO 4 Dry, filter with suction, concentrate to quick dryness, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com