Application of saikoside compounds in preparation of drug for treating neurodegenerative diseases
A neurodegenerative and saikosaponin technology, applied in the field of medicine, can solve the problems of special parts, complex pathogenesis of ND, and lack of therapeutic drugs, etc., and achieve the effect of enhancing nest building ability, firm nest, and obvious protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The statistical results of the effects of the saikosaponin series compounds of Example 1 and Example 2 on the release of microglial inflammatory factors NO and ROS are shown in Table 1;
[0033] Example 1: The effect of saikosaponin on LPS-induced NO release from microglial cells was investigated by Griess colorimetry;
[0034] Cell line: mouse microglial cell line BV-2;
[0035] Drugs: LPS; saikosaponin a; saikosaponin b1, b2, c, d; MINO.
[0036] method:
[0037] (1) Culture of mouse microglial cell line BV-2:
[0038] The cell culture solution was prepared based on DMEM medium, containing 10% FBS, 100 U penicillin and 100 U streptomycin, and 50 μM 2-mercaptoethanol (both final concentrations). at 5% CO 2 , under the condition of 37 ℃, the BV-2 microglial cells were divided into about 4×10 5 The cell density of cells / ml was cultured in a cell culture incubator, and the growth of the cells was observed regularly. When the area of the cell adherence accounted for...
Embodiment 3
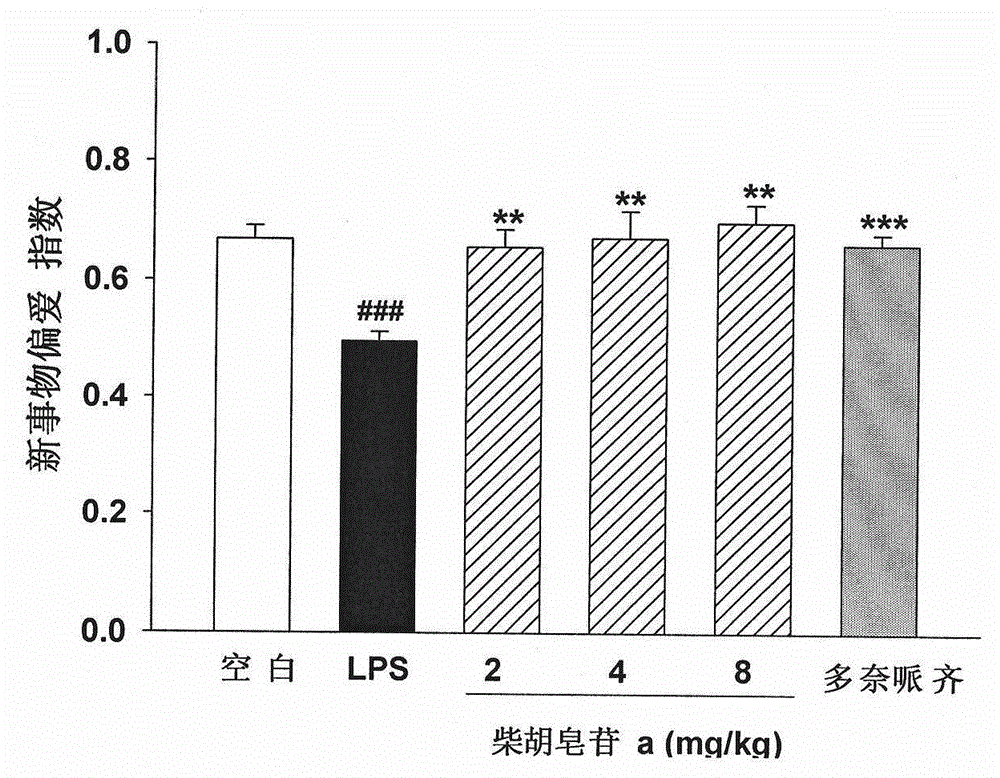
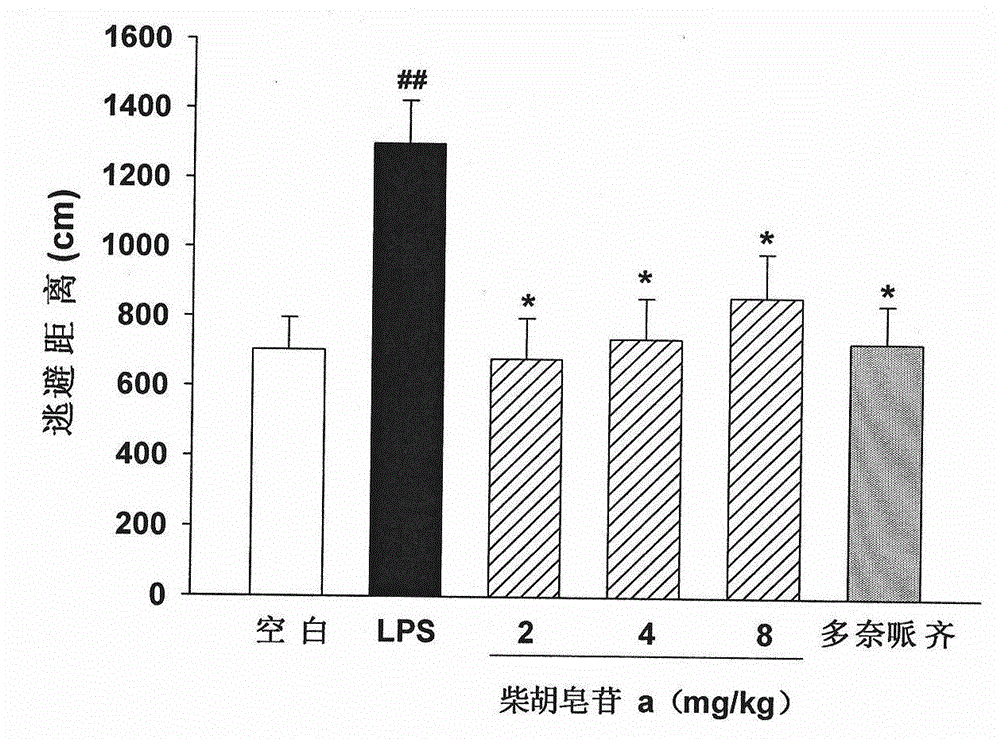
[0051] Embodiment 3: Novel Object Recognition (NovelObjectRecognition, NOR) experiment investigates the impact of saikosaponin a on the novel object discrimination ability of LPS-induced learning and memory impairment mice; the structure is as figure 1 Shown:
[0052] Material animal: ICR male mouse, 18-22g;
[0053] Drugs: LPS; saikosaponin a; donepezil hydrochloride;
[0054] Methods: ICR mice were randomly divided into 6 groups, 12 in each group: blank control group, model control (LPS) group, saikosaponin a high, medium and low dose group and positive control group (donepezil hydrochloride). Animals in each group were lightly anesthetized with chloral hydrate, fixed their heads, and injected into the lateral ventricle. Model group, saikosaponin a low dose (2 mg / kg) group, saikosaponin a medium dose (4 mg / kg) group, saikosaponin a high dose (8 mg / kg) group and positive control group (5 mg / kg) LPS solution (5 mg / ml, 3 μl / mouse) was injected, and mice in the blank control ...
Embodiment 4
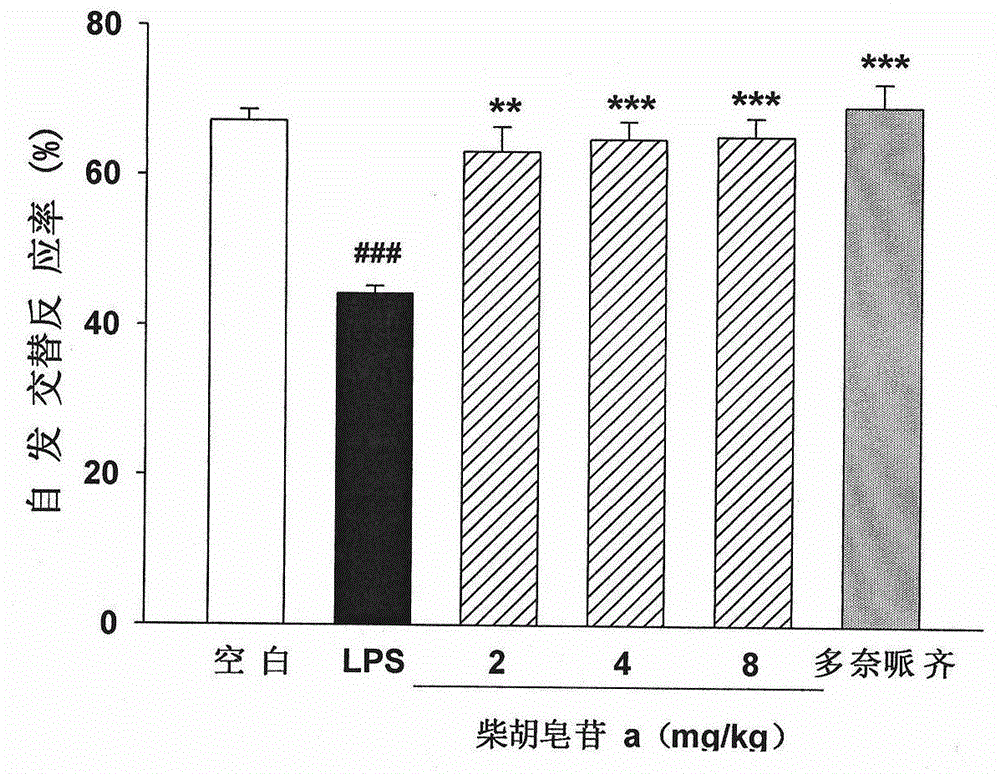
[0057] Example 4: Y maze experiment to investigate the effect of saikosaponin a on the working memory ability of LPS-induced learning and memory impairment mice; the results are as follows figure 2 Shown:
[0058] Material animal: ICR male mouse, 18-22g;
[0059] Drugs: LPS; saikosaponin a;
[0060] Methods: ICR mice were randomly divided into 6 groups, 12 in each group: blank control group, model control (LPS) group, saikosaponin a high, medium and low dose group and positive control group (donepezil hydrochloride). Animals in each group were lightly anesthetized with chloral hydrate, fixed their heads, and injected into the lateral ventricle. Model group, saikosaponin a low dose (2 mg / kg) group, saikosaponin a medium dose (4 mg / kg) group, saikosaponin a high dose (8 mg / kg) group and positive control group (5 mg / kg) LPS solution (5 mgl / ml, 3 μl / mouse) was injected, and the mice in the blank control group were injected with an equal volume of normal saline. From the next ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com