Uses of icariin or icariside II in prevention and treatment of kidney diseases
A technology of icariin and icariin is applied in the fields of urinary system diseases, medical preparations containing active ingredients, organic active ingredients, etc., and can solve the problem of unable to block the pathological changes of chronic kidney disease.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 icariin and icariin II
[0051] Icariin and Epimedium were prepared according to the method of XiaQ et al. Inosin II.
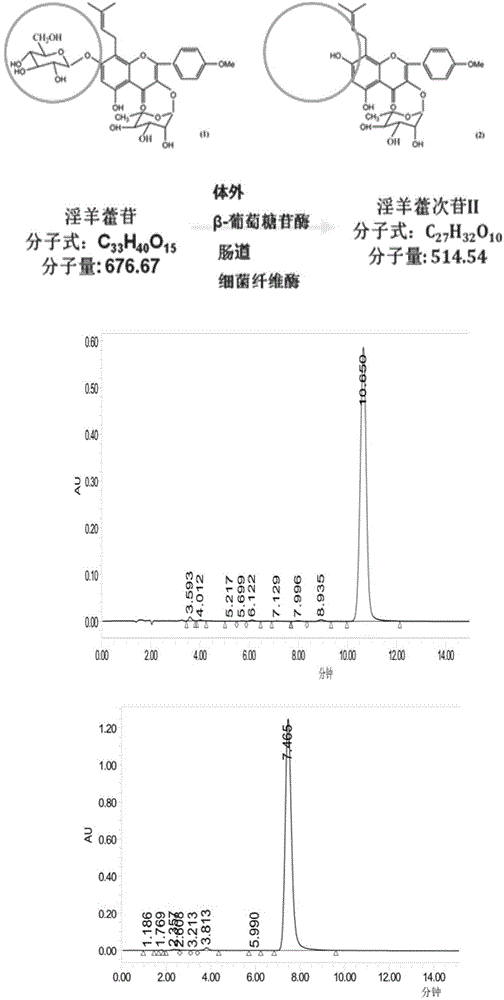
[0052] Preparation of icariin: extraction with ethanol and purification with macroporous resin to extract and separate icariin from Epimedium. A large amount of icariin (Icariin, ICA) can be obtained. Its purity was 98.0% by HPLC analysis.
[0053] Preparation of Icariside II:
[0054] In vitro, the icariin in Epimedium was converted into icariside Ⅱ (Icariside Ⅱ, ICA Ⅱ) by glycosidase (β-glucosidase) fermentation, and purified according to the literature method. Utilize HPLC to analyze its purity to be 98.0% (as figure 1 ).
[0055] The prepared icariin and icariin II were used in the following examples.
Embodiment 2
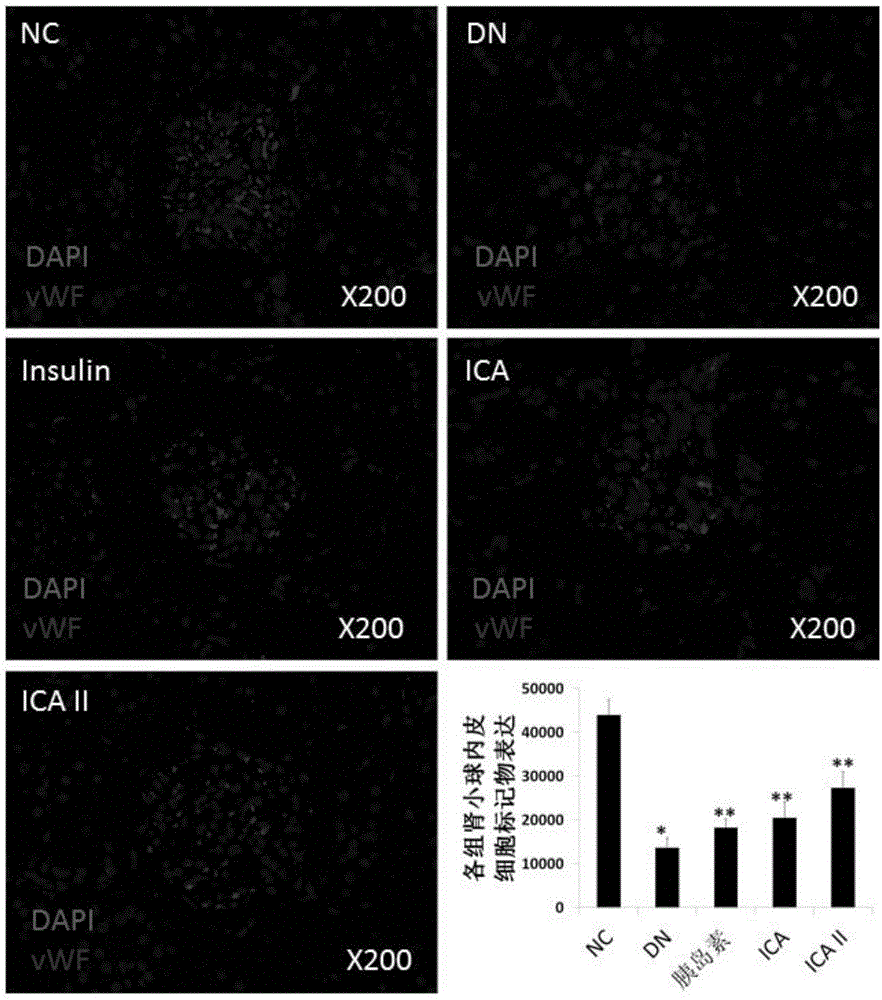
[0056] Example 2 Prevention and treatment effect of icariside Ⅱ on chronic kidney disease caused by diabetes
[0057] 1. Establishment of renal endogenous stem cell marker chronic kidney disease rat model
[0058] Chronic kidney disease is mainly caused by diabetes, hypertensive renal arteriosclerosis, glomerulonephritis, and nephrotoxic drugs. Among them, the chronic kidney disease model in diabetic rats is one of the most commonly used and reasonable models.
[0059]The inventor used 50 SD rats and injected EdU (thymidine analog) (50 mg / kg) (Invitrogen, Carlsbad, CA, USA) intraperitoneally on the second day after birth to prepare experiments carrying EdU(+)-LRCs Rats), raised for 8 weeks, were randomly divided into healthy control group NC (n=10), and the remaining rats were fasted by intraperitoneal injection of STZ (60mg / kg, ip) (SigmaChemicalCo, StLouis, Missouri) to establish a diabetes model group (n =40), 72 hours after STZ injection, the blood glucose was detected t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com