Urapidil composition freeze-dried powder injection for treating hypertension
A technology of freeze-dried powder injection and urapidil, applied in the field of medicine, can solve the problems of difficult storage, toxicity to patients, poor stability, etc., and achieve the effects of low content of insoluble particles, improved fluidity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of urapidil crystals
[0029] (1) Mix acetone, ethanol, and N,N-dimethylformamide into a mixed solvent, and the volume ratio of acetone, ethanol, and N,N-dimethylformamide is 2:4:1;
[0030] (2) Take urapidil raw material, dissolve it in a mixed solvent of acetone, ethanol, N,N-dimethylformamide whose volume is 6 times the weight of uradil in step (1), heat up to 35°C, Stir until completely dissolved to obtain urapidil solution;
[0031] (3) Under the condition of a stirring speed of 560r / min, add the urapidil solution in step (2) into acetone whose volume is 5 times the weight of urapidil, mix to form a suspension, and mix at 10°C / min Cool down to -10°C at a faster rate;
[0032] (4) Perform suction filtration, wash the filter cake, and then vacuum-dry the filter cake to obtain a crystalline powder, which is the urapidil compound.
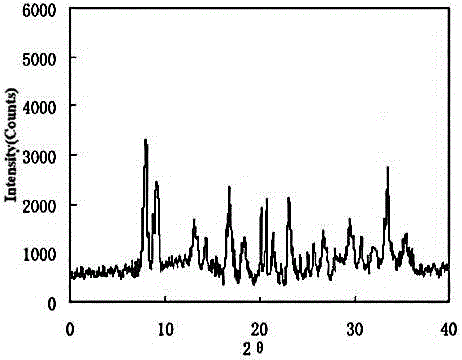
[0033] The X-ray powder diffraction pattern obtained by measuring the obtained urapidil crystal using Cu-Kα ra...
Embodiment 2
[0034] Example 2: Preparation of urapidil freeze-dried powder injection
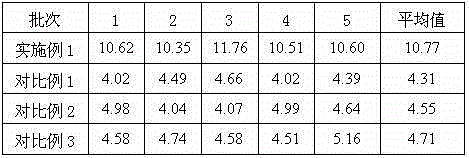
[0035] Prescription: 3 parts by weight of the urapidil crystal form compound prepared in Example 1 and 1.5 parts by weight of low molecular weight dextran.
[0036] Preparation:
[0037] Take the urapidil compound of the present invention, stir and dissolve it with water for injection, add the prescribed amount of low-molecular dextran, adjust the pH value to 5.0-6.5, and then stir until the pH remains constant, then add water for injection until the volume of the solution is uradil 150 times its weight, then coarsely filter with activated carbon, pass through 1.0μm, 0.45μm, 0.22μm microporous membranes in turn to sterilize and filter, filter into a sterile room, measure pH and content after passing, fill, press half stopper , put it into a freeze-drying box that has been cooled to -40°C, freeze-dry, press the plug out of the box, and roll the cover.
[0038] Described freeze-drying is:
[0039] Pre...
Embodiment 3
[0040] Example 3: Preparation of urapidil freeze-dried powder injection
[0041] Prescription: 3 parts by weight of the urapidil crystal form compound prepared in Example 1 and 2.25 parts by weight of low molecular weight dextran.
[0042] Preparation:
[0043]Take the urapidil compound of the present invention, stir and dissolve it with water for injection, add the prescribed amount of low-molecular dextran, adjust the pH value to 5.0-6.5, and then stir until the pH remains constant, then add water for injection until the volume of the solution is uradil 150 times its weight, then coarsely filter with activated carbon, pass through 1.0μm, 0.45μm, 0.22μm microporous membranes in turn to sterilize and filter, filter into a sterile room, measure pH and content after passing, fill, press half stopper , put it into a freeze-drying box that has been cooled to -40°C, freeze-dry, press the plug out of the box, and roll the cover.
[0044] Described freeze-drying is:
[0045] Pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com