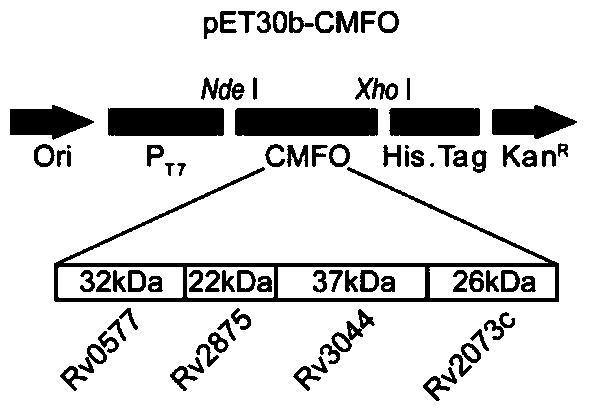
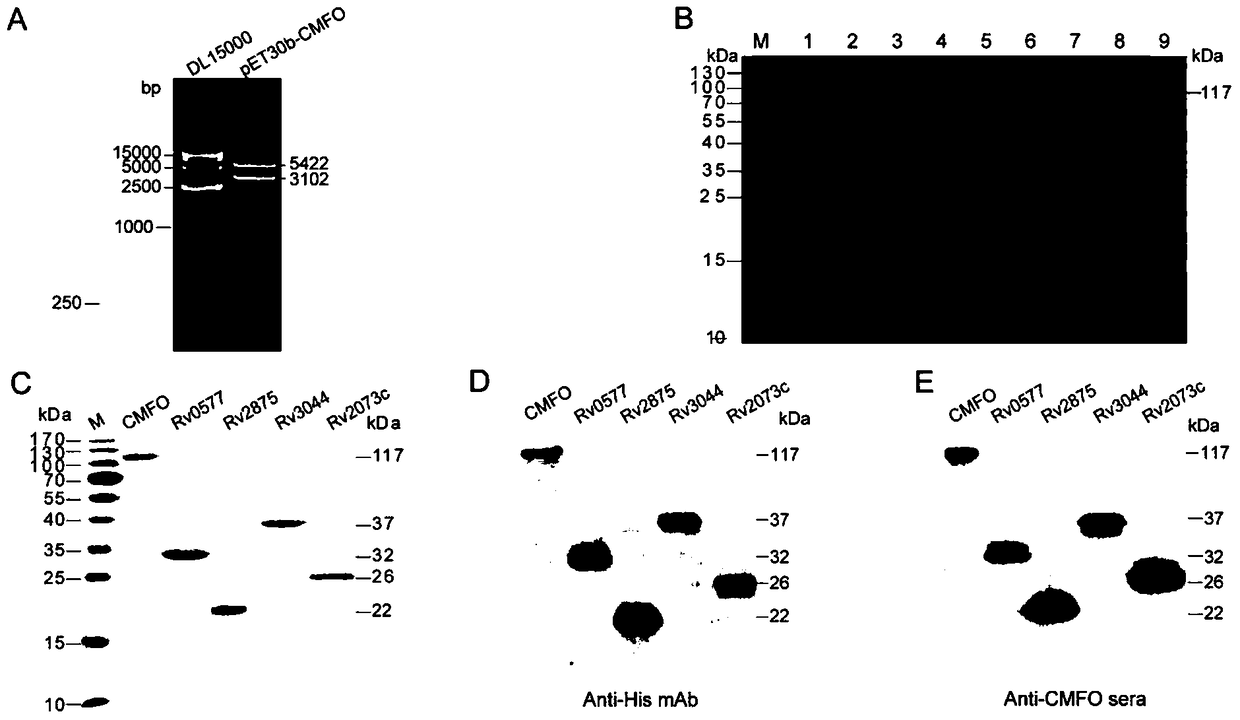
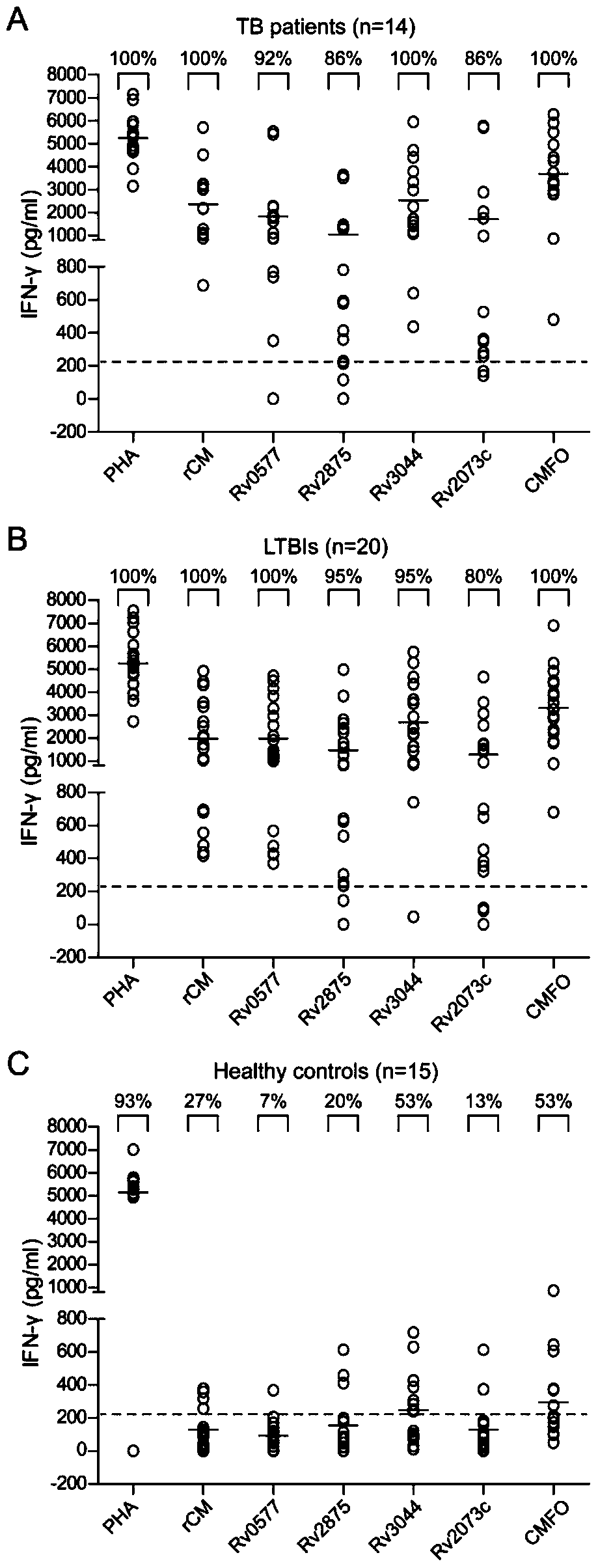
A kind of fusion protein cmfo and its application
A fusion protein and protein technology, applied to fusion protein CMFO and its application field, can solve the problem of unsatisfactory effect of preventing latent infection, and achieve the effect of preventing latent infection and reducing the risk of vaccination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Design and synthesis of fusion gene CMFO sequence and prokaryotic expression primers thereof
[0038] According to the whole gene sequence of M.tb H37Rv, the coding sequences of Rv0577, Rv2875, Rv3044 and Rv2073c were selected. Use Signalp software to predict and search the UniProtKB database to see if the sequence has a signal peptide, and then use DNAMAN software to analyze the restriction site and amino acid sequence. The following principles should be followed when splicing 4 separate target genes: 1) If there is a signal peptide, the signal peptide should be removed, including the original start codon, and the stop codon should be removed at the same time (add TAA after the last spliced gene sequence ); 2) The gene sequence without signal peptide retains the start codon sequence. If the start codon is GTG, because the original expression product is Met instead of Val, GTG needs to be corrected to ATG. The splicing sequence of the fusion protein gene i...
Embodiment 2
[0040] Example 2 Construction of Fusion Protein CMFO Prokaryotic Expression Vector and Protein Purification
[0041] 1. Acquisition of the target gene:
[0042] (1) Primer design: Design primers based on the full sequence of the fusion gene and the multiple cloning site on the prokaryotic expression vector pET30b(+). The primer sequence was synthesized by Shanghai Yingjun Biological Company, and the primer was diluted to 100 pmol / μl according to the instructions. Store at -20°C for later use. The underline in the following primer sequences indicates the enzyme recognition site.
[0043] CMFO-Fwd:
[0044] (Nde I)-TTC CATATG CCCAAGAGAAGCGAATACAGGCAA
[0045] CMFO-Rev:
[0046] (Xho I)-AT CTCGAG TCGCGGCATCCTGCGCCAGACGAAC
[0047] (2) PCR reaction system:
[0048]
[0049] (3) PCR reaction conditions:
[0050] 95℃5min; 94℃1min, 64℃1min and 72℃3min, 30cycles; 72℃10min; 4℃forever
[0051] 2. Construction of recombinant plasmids:
[0052] The target gene CMFO with Nd...
Embodiment 3
[0062] A tuberculosis subunit vaccine, containing the fusion protein CMFO prepared in Example 2, its concentration is respectively 0.1mg / ml, 0.5mg / ml, 1mg / ml, respectively mixed with DMT adjuvant, equal volume, conventional mechanical vibration or stirring , mixed to form a homogeneous suspension.
[0063] The preparation method of described tuberculosis subunit vaccine is as follows:
[0064] Pipette 100 μl of fusion protein solution with a working concentration of 0.2 mg / ml and 100 μl of prepared DMT adjuvant into a sterile EP tube, close the tube cap tightly, and oscillate on a vortex shaker for 2 to 3 minutes at intervals to form a uniform milk solution, that is, the tuberculosis subunit vaccine CMFO / DMT was prepared.
[0065]The DMT vaccine adjuvant contains 2mg / ml dimethyl dioctadecyl ammonium (DDA), 0.4mg / ml monophosphoryl lipid (MPL) A, and 0.4mg / ml trehalose (TDB), which is In liposomal form. The trehalose is artificially synthesized 6,6'-dimycolic acid.
[0066] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com