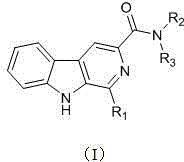
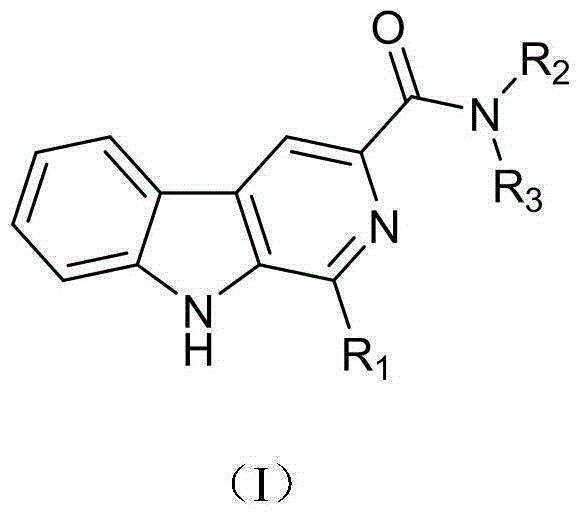
Harmine compound and applications of harmine compound in prevention and control of peronophthora litchi chen disease
A technology of humaline and compounds, applied in the application field of pesticide fungicide drugs, to achieve the effects of small molecular weight, inhibitory effect, and inhibited growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 N-ethyl-1-phenyl-β-carboline-3-carboxamide (F1)
[0031] 1. Step A: Synthesis of 1-phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (2)
[0032] Weigh 20.4g L-tryptophan (0.1mol) into a three-necked flask equipped with an electric heating mantle and an electric stirrer, add 100mL glacial acetic acid while stirring, and add 12.25g benzaldehyde (0.12mol) at the same time. After stirring at room temperature for 15 minutes, the temperature is increased. Reacted at 80~100℃ for 10h, TLC traced to the disappearance of tryptophan, stopped heating, cooled to room temperature and precipitated, filtered, and the filtrate was concentrated under reduced pressure to remove excess acetic acid and water to obtain a large amount of light brown flakes. After washing with water, a white product, namely 1-phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid was obtained with a yield of 85%.
[0033] 2. Step B: Synthesis of 1-phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic ...
Embodiment 2
[0043] Example 2N,N-Diethyl-1-phenyl-β-carboline-3-carboxamide (F2)
[0044] 1. The operation is the same as in Example 1, except that in step E, diethylamine is used instead of ethylamine.
[0045] 2. The product monitoring data are as follows: Yield: 57.10%; Melting point: 210-212°C; IR (KBr) cm -1 :3449(ν N-H ),2918,2851(ν C-H ),1623(ν C=O ),1022(ν C-N ); 1 H-NMR(600MHz,DMSO)δ11.71(1H,s,N(9)H,c), 8.42(1H,s,C(4)H,c), 8.35(1H,d,J=7.8Hz ,C(5)H,c),8.02(2H,d,J=7.4Hz,Ph(2,6)H),7.67(1H,d,J=8.2Hz,C(8)H,c), 7.63(2H,t,J=7.6Hz,Ph(3,5)H),7.56(2H,m,J=19.5,7.5Hz,C(7)H,c,Ph(4)H),7.29( 1H, t, J = 7.4 Hz, C (6) H, c), 3.50 (4H, m, J = 13.7, 6.8 Hz, CH 2 ),1.23-1.18(6H,m,CH 3 ).
Embodiment 3
[0046] Example 3 N-isopropyl-1-phenyl-β-carboline-3-carboxamide (F3)
[0047] 1. The operation is the same as in Example 1, except that in step E, isopropylamine is used instead of ethylamine.
[0048] 2. The product monitoring data are as follows: Yield: 47.27%; Melting point: 188-191°C; IR (KBr) cm -1 :3430(ν N-H ),2924,2850CH,CH 3 ,1647(ν C=O ),1252(ν C-N ); 1 H-NMR(600MHz,DMSO)δ11.82(1H,s,N(9)H,c),8.82(1H,s,C(4)H,c),8.41(1H,d,J=7.9Hz ,CO-NH), 8.28(1H,d,J=8.3Hz,C(5)H,c),8.12(2H,d,J=7.3Hz,Ph(2,6)H),7.67(3H, dd,J=17.0,8.1Hz,C(8)H,c,Ph(3,5)H),7.59(2H,dd,J=17.0,7.7Hz,C(7)H,c,Ph(4 )H),7.35-7.23(1H,m,C(6)H,c), 4.20(1H,m,J=13.4,6.7Hz,CH), 1.26(6H,d,J=6.6Hz,CH 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com