The synthetic method of hexamidine
A synthesis method and a technology of hexamidine are applied in the field of organic synthesis, and can solve the problems of large amount of waste acid, low yield of intermediate II, low industrial value, etc., and achieve the acceleration of cyano addition reaction, the provision of production safety, Avoid volatile effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
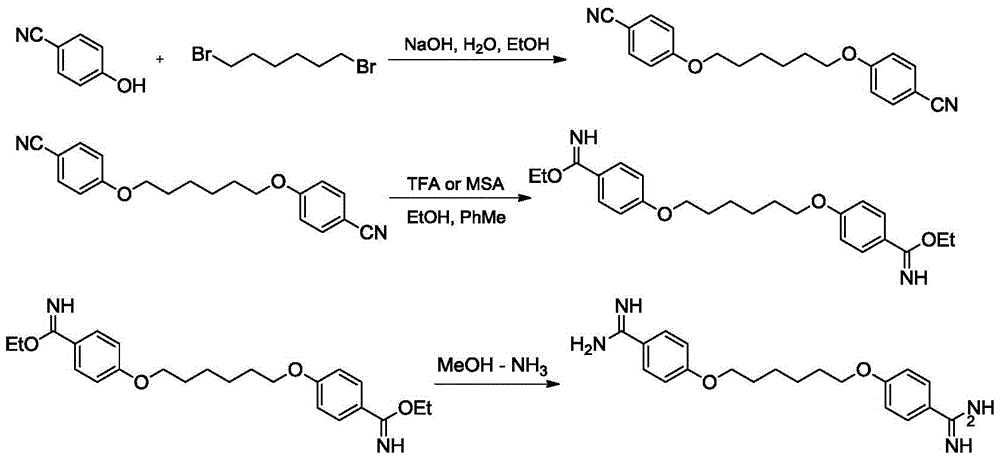
[0032] (1) Synthesis of 1,6-(p-cyanophenyl) hexylene diether (intermediate Ⅰ):
[0033] In a 2L three-necked flask equipped with stirring, a thermometer, a dropping funnel, and a reflux condenser, add 23.3g (0.56mol) of sodium hydroxide and 240ml of water in sequence, stir to dissolve completely, and then cool to room temperature; dropwise add Contain 66.7g (0.56mol) of 4-cyanophenol in 267ml of ethanol solution, control the rate of addition, and keep the temperature at 25-30°C; after the dropwise addition, slowly increase the temperature, react at 80°C for 2 hours, and cool to room temperature; dropwise add 65.3g (0.27mol) 1,6-dibromohexane, reflux (80-85°C) for 8-10h, cool to 0°C, stir for 2-3h, and filter with suction to obtain 98g of intermediate Ⅰ white solid product, add the wet product to In 417ml of ethanol, heated and stirred to 75°C for reflux reaction for 2-3h, cooled, filtered at about 50°C, dried the filter cake, weighed to obtain 70.01g, yield 81%.
[0034] (2) Sy...
Embodiment 2
[0040] (1) Synthesis of 1,6-(p-cyanophenyl) hexylene diether (intermediate I): same as Example 1(1)
[0041] (2) Synthesis of 1,6-(p-iminocarbamate ethylphenyl) hexamethylene diether (intermediate II):
[0042] Add 70g (0.22mol) of intermediate I product, 400ml ethanol, and 1260ml toluene to the reaction flask, add 67g methanesulfonic acid (4.8% of the total amount of the reaction system) under stirring under 10°C, and heat up at 39°C for 43h. It was detected by TLC that the raw material of intermediate I had completely disappeared. After the reaction was completed, it was cooled to below 10°C, filtered, and dried to obtain 86.4 g of crude product. Yield 95.2%. The product is identified as the expected structure by proton nuclear magnetic spectrum, and the proton spectrum data are the same as those in Example 1 (2).
[0043] (3) Synthesis of hexamidine (4-(6-(4-formamidinephenoxy)hexyloxy)benzamidine):
[0044] With embodiment 1 (3). The total yield of the above-mentioned ...
Embodiment 3
[0046] (1) Synthesis of 1,6-(p-cyanophenyl) hexylene diether (intermediate I): same as Example 1(1)
[0047] (2) Synthesis of 1,6-(p-iminocarbamate ethylphenyl) hexamethylene diether (intermediate II):
[0048] Add 70g (0.22mol) of intermediate I product, 400ml of ethanol, and 1260ml of toluene to the reaction flask, add 164g of trifluoroacetic acid (10% of the total amount of the reaction system) under stirring under 10°C, raise the temperature at 40°C for 43h, TLC It was detected that the raw material of intermediate I had completely disappeared, and the reaction was completed, cooled to below 10°C, filtered, and dried to obtain 88.9 g of crude product. Yield 98%. The product is identified as the expected structure by proton nuclear magnetic spectrum, and the proton spectrum data are the same as those in Example 1 (2).
[0049] (3) Synthesis of hexamidine (4-(6-(4-formamidinephenoxy)hexyloxy)benzamidine):
[0050] With embodiment 1 (3). The total yield of the above-menti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com