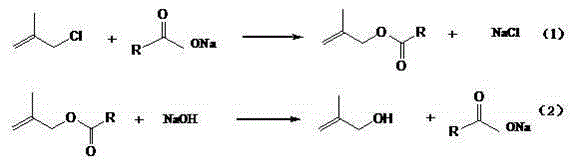
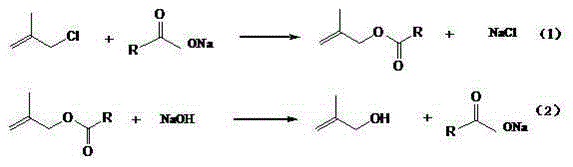
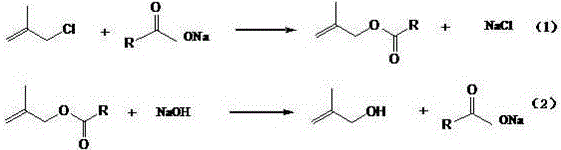
Synthetic method of 2-methallyl alcohol
A technology of methallyl alcohol and a synthesis method, which is applied in the field of synthesis of 2-methallyl alcohol, can solve the problems of equipment corrosion, large amount of waste water and high hydrogenation pressure, achieves improved product yield, reduced condition requirements, The effect of prolonging the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Esterification reaction: In the autoclave, put 588g 2-methallyl chloride, 532.8g anhydrous sodium acetate (the molar ratio of 2-methallyl chloride and anhydrous sodium acetate is 1.0:1.0) and 10.6 g tetrabutylammonium chloride, seal the reaction kettle, start stirring and raise the temperature to 100°C, with the increase of temperature, the pressure is up to 0.3MPa, and keep warm for 20h. After the reaction was completed, the material was transferred to a still, and 739.6 g of the organic phase was distilled out. According to gas chromatography analysis, the content of methacryl acetate was 96.2%, and the content of 2-methallyl chloride was 3.8%. The solid in the still is mainly sodium chloride 391.8g, wherein the sodium chloride content is 97.2%, and the moisture is 0.4%. The organic phase was rectified under the conditions of -0.040MPa at the top of the tower and 70°C at the top of the tower to obtain 669.7g of refined methallyl acetate with a methallyl chloride conte...
Embodiment 2
[0040] Esterification reaction: In the autoclave, put 588g 2-methallyl chloride, 410g anhydrous sodium acetate (the molar ratio of 2-methallyl chloride and anhydrous sodium acetate is 1.3:1) and 6.1g Tetrabutylammonium chloride, seal the reaction kettle, start stirring and raise the temperature to 120°C. With the increase of temperature, the pressure is up to 0.4MPa, and the temperature is kept for 15h. After the reaction was complete, the material was transferred to a distillation pot, and 706.0 g of the organic phase was distilled out. According to gas chromatography analysis, the content of methacryl acetate was 80.2%, and the content of 2-methallyl chloride was 19.8%. The solid in the still is mainly sodium chloride 298.1g, wherein the sodium chloride content is 98.2%, and the moisture is 0.3%. The organic phase was rectified under the conditions of -0.040MPa at the top of the tower and 70°C at the top of the tower to obtain 541.8g of refined methallyl acetate with a metha...
Embodiment 3
[0043] Esterification reaction: In the autoclave, put 588g 2-methallyl chloride, 355.2g anhydrous sodium acetate (the molar ratio of 2-methallyl chloride and anhydrous sodium acetate is 1.5:1) and 3.6 g tetrabutylammonium chloride, seal the reaction kettle, start stirring and raise the temperature to 140°C. With the increase of temperature, the pressure is up to 0.5MPa, and the temperature is kept for 10h. After the reaction was completed, the material was transferred to a still, and 689.5 g of the organic phase was distilled out. According to gas chromatography analysis, the content of methacryl acetate was 71.6%, and the content of 2-methallyl chloride was 28.4%. The solid in the still is mainly 257.3g of sodium chloride, wherein the content of sodium chloride is 98.5%, and the moisture is 0.3%. The organic phase was rectified at -0.040MPa at the top of the tower and the temperature at the top of the tower was 70°C to obtain 469.2g of refined methallyl acetate with a methall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com