Rapid fluorescence PCR detection kit for Chlamydia trachomatis
A technology of Chlamydia trachomatis and detection kit is applied in the field of fluorescent PCR kits and early clinical diagnosis of Chlamydia trachomatis, which can solve the problems of unsuitable basis for clinical diagnosis and diagnosis, unable to meet the needs of rapid diagnosis and treatment, poor detection accuracy, etc. Risk of contamination, fast detection and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 kit
[0031] 1. Design and synthesis of kit-specific primers
[0032] According to the CT sequence published online (GenBank: CP006677.1), the Chlamydia trachomatis PCR primers were designed for the conserved Chlamydia trachomatis gene trpB fragment using the biology software Primer Premier 5 software and Oligo 7 software, and the software Mega4 and NCBI online Blast were used for the design Sequence alignment ensures the specificity and rationality of each pair of primers. The primers were synthesized by PAGE Bioengineering (Dalian) Co., Ltd. and purified by PAGE. The nucleotide sequences of the kit-specific primers are as follows:
[0033] Forward primer: 5'-CTATACAGAATCTAATGGCGGAATG-3',
[0034] Reverse primer: 5'-TAGCAAGCAAACACTGACCTTG-3',
[0035] 2. Design and preparation of CT plasmid positive quality control products
[0036]A pair of amplification primers are newly designed on the periphery of the sequence amplified by the ...
Embodiment 2
[0050] The usage method of embodiment 2 kit
[0051] 1. Sample requirements
[0052] a. Sample collection: A special swab sterilized by autoclaving is inserted into the male urethra or female cervix 1-2 cm, rotated once, take it out after staying for about 10 seconds, put the cotton swab head into 1ml sterile physiological The saline sample is stored in a cryopreservation tube, and the swab is cut off from the neck, and the swab head is immersed in the saline, and rinsed repeatedly to obtain the specimen rinsing solution.
[0053] b. Storage: The obtained specimen rinse solution should be stored at -20°C, and samples that cannot be detected within 24 hours should be stored below -70°C. Specimens should be stored separately in special counters or warehouses.
[0054] c. Transportation: Use curlers with ice packs or foam boxes with ice packs for transportation.
[0055] 2. Sample preparation
[0056] Take 500 μl of specimen washing solution, centrifuge at 10,000 rpm for 2 mi...
Embodiment 3
[0073] The performance evaluation of embodiment 3 kits
[0074] 1. Sensitivity experiment
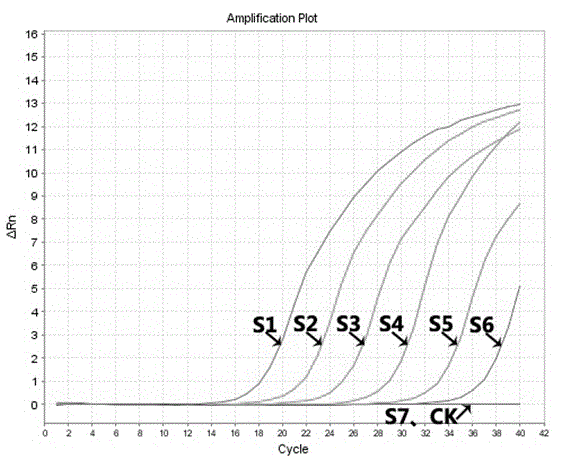
[0075] CT sensitivity reference product (5.0×10 5 copies / μl), consisting of a plasmid containing the CT target fragment, using TE buffer, the above-mentioned sensitivity reference product was diluted 10 times to obtain the following gradient solution: S1 is 5.0×10 5 copies / μl, S2 is 5.0×10 4 copies / μl, S3 is 5.0×10 3 copies / μl, S4 is 5.0×10 2 copies / μl, S5 is 5.0×10copies / μl, S6 is 5.0copies / μl, S7 is 5.0×10 -1 Copies / μl is used as a sensitivity reference product, and the sensitivity reference product is used for detection. The minimum detection amount is 5.0copies / μl, that is, 10copies / reaction. For the experimental results, see figure 1 . figure 1 It shows that the CT sensitivity reference product of the kit is at 1.0×10 6 There is a good linear relationship between copies / μl and 1.0×10copies / μl, and the minimum detection amount of the kit is 5.0copies / μl, that is, 10 copies / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com