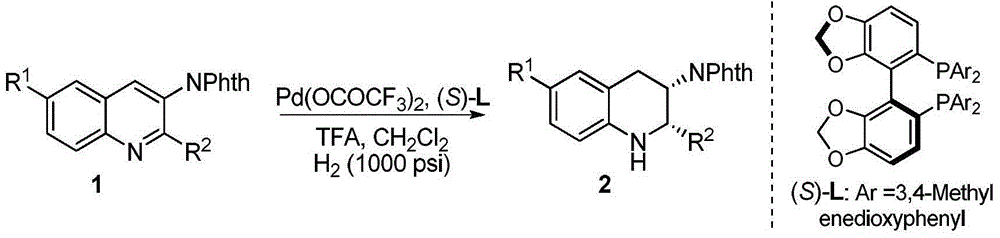
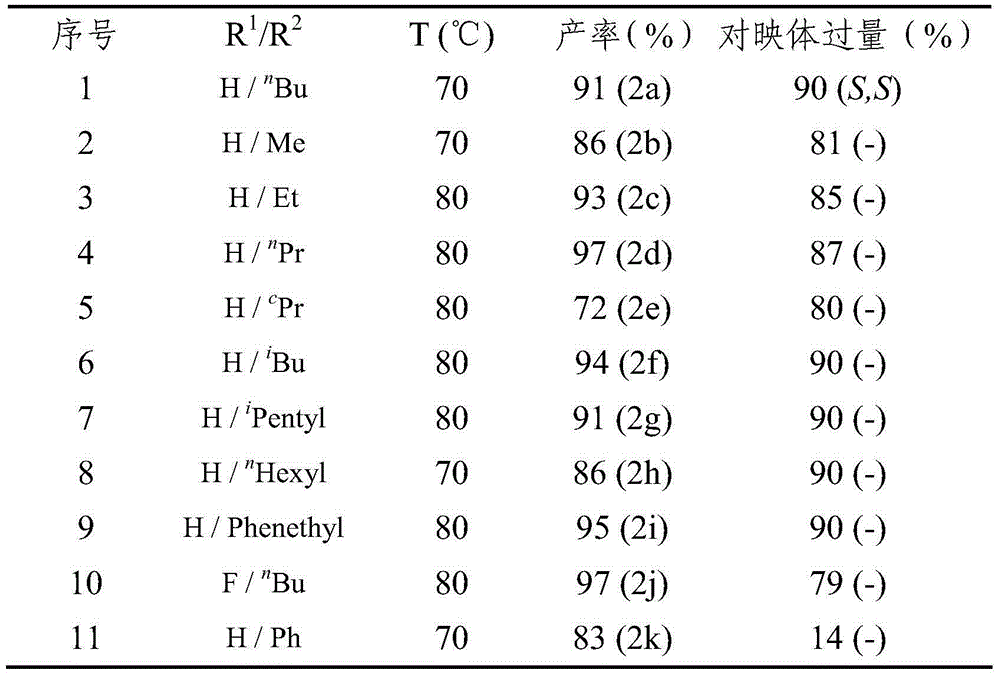
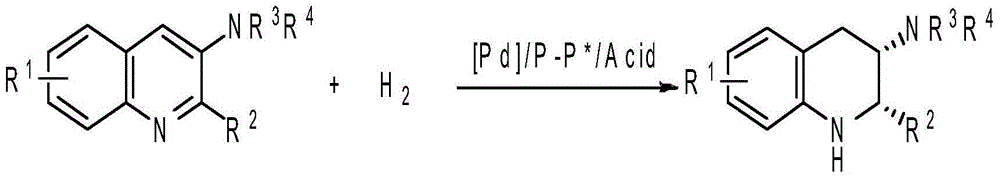Method for synthesizing chiral cyclohexanediamine through asymmetric hydrogenation of palladium-catalyzed quinoline-3-amine
A chiral exocyclic amine, catalyzing quinoline technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of strong coordination ability, aromatic amines Low activity, catalyst poisoning and other problems, to achieve the effect of complete reaction, convenient preparation and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of various chiral exocyclic amine compounds by palladium-catalyzed asymmetric hydrogenation of quinoline-3-amine
[0029] Put palladium trifluoroacetate (0.005 mmol) and (S)-L (0.006 mmol) into the reaction flask, add 1 ml of acetone after nitrogen replacement, stir at room temperature for 1 hour, then concentrate in vacuo to remove acetone to obtain a catalyst. In the glove box, the prepared catalyst was dissolved in dichloromethane, and then transferred to another reaction flask with substrate (0.10 mmol) and trifluoroacetic acid (0.06 mmol) in advance, sharing 4 ml of dichloromethane. After stirring for 5 minutes, the reaction bottle was put into a stainless steel autoclave, hydrogen gas (1000 psi) was introduced, and the reaction was carried out at 70° C. (or 80° C.) for 18 hours. Slowly release the hydrogen gas, stir with 5 ml of saturated sodium bicarbonate at room temperature for 10 minutes to make it alkaline, remove the activator acid, extr...
Embodiment 2
[0045] Embodiment 2: the synthesis of a kind of substance P antagonist 4
[0046] Under the protection of nitrogen, add hydrazine hydrate (2.00 mmol) to the ethanol solution dissolved in 2k (0.10 mmol), and then react at 60°C. After the reaction is complete, spin off the solvent, and use 10 mL dichloromethane and 10 mL Water solvent, liquid separation, aqueous phase extracted with dichloromethane, combined organic phases, dried over anhydrous sodium sulfate, filtered, spin-dried, combined organic phases, dried over anhydrous sodium sulfate, filtered and then rotary evaporated to remove solvent, column chromatography Pure product 3 was isolated.
[0047] Under nitrogen protection, compound 3 (0.10 mmol) and NaBH (OAc) 3 (0.30 mmol) was dissolved in 2 mL of 1,2-dichloroethane, and then a solution of o-methoxybenzaldehyde (0.11 mmol) in 1 mL of 1,2-dichloroethane was added dropwise, and then heated at 50°C After the reaction is complete, use saturated NaHCO 3 The solution quen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com