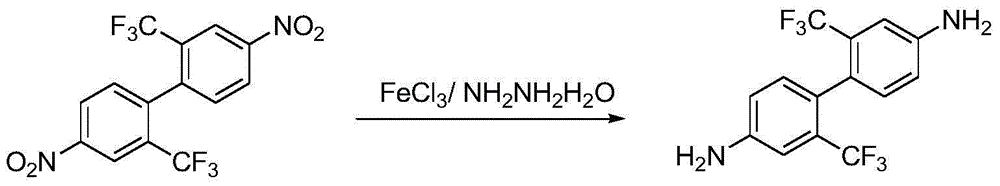Preparation method of 2,2'-bistrifluoromethyl-4,4'-diaminobiphenyl
A bistrifluoromethyl and diaminobiphenyl technology is applied in the preparation of amino compounds, the preparation of organic compounds, chemical instruments and methods, etc. It can solve problems such as difficulties in industrial production, inability to use large quantities, explosions, etc., and achieve solvent-free Effects of recycling, easy control of reaction, and simple investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 76kg of 2,2'-bistrifluoromethyl-4,4'-dinitrobiphenyl to the reaction kettle and dissolve it in 608kg of methanol, then add 32kg of anhydrous ferric chloride, and heat up to 50°C under stirring. Add dropwise 95kg of 80% hydrazine hydrate (the molar ratio is: 2,2'-bistrifluoromethyl-4,4'-dinitrobiphenyl:ferric chloride:80% hydrazine hydrate:methanol=1:1.09: 8:80), the dropping temperature remained basically unchanged, after the dropping was completed, the temperature was raised to reflux, and after the reaction of the raw materials was completed, the temperature was lowered to room temperature, filtered, the filtrate was depressurized and the solvent was recovered, and the crystal was precipitated, cooled and filtered, and dried to obtain 2,2' - Bistrifluoromethyl-4,4'-diaminobiphenyl 55.3 kg, off-white solid, yield 91%.
Embodiment 2
[0018] Add 76kg 2,2'-bistrifluoromethyl-4,4'-dinitrobiphenyl to the reaction kettle and dissolve it in 570kg methanol, then add 31.4kg anhydrous ferric chloride, and heat up to 45 ℃, drop 71kg 80% hydrazine hydrate (molar ratio: 2,2'-bistrifluoromethyl-4,4'-dinitrobiphenyl:ferric chloride:80% hydrazine hydrate:methanol=1: 1.02:6:75), the dropping temperature remains basically unchanged, after the dropwise addition is completed, the temperature is raised to reflux, and after the reaction of the raw materials is complete, the temperature is lowered to room temperature, filtered, the filtrate is decompressed and the solvent is recovered, the crystal is precipitated, and the temperature is filtered, and dried to obtain 2, 43.8 kg of 2'-bistrifluoromethyl-4,4'-diaminobiphenyl, off-white solid, yield 72%.
Embodiment 3
[0020] Add 76kg 2,2'-bistrifluoromethyl-4,4'-dinitrobiphenyl to the reaction kettle and dissolve it in 650kg of methanol, then add 35.7kg of anhydrous ferric chloride, and heat up to 50 ℃, drop 115kg 80% hydrazine hydrate (the molar ratio is: 2,2'-bistrifluoromethyl-4,4'-dinitrobiphenyl: ferric chloride: 80% hydrazine hydrate: methanol=1: 1.16:10:85), the dropping temperature remains basically unchanged, after the dropwise addition is completed, the temperature is raised to reflux, and after the reaction of the raw materials is complete, the temperature is lowered to room temperature, filtered, the filtrate is decompressed and the solvent is recovered, the crystal is precipitated, and the temperature is filtered, and dried to obtain 2, 56.1 kg of 2'-bistrifluoromethyl-4,4'-diaminobiphenyl, off-white solid, yield 92.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com